Tröger's Base
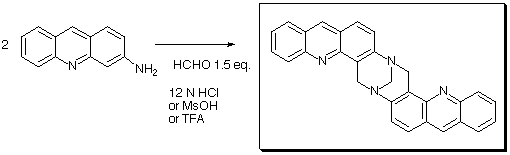
Tröger's base analogue is obtained with high selectivity as determined
by HPLC and in 70% yield after purification in either solvent used with a
stochiometric amount of paraformaldehyde.
The X-ray analysis was performed by Dr C. Courseille (Université Bordeaux I)
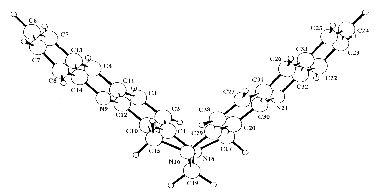
The X-ray analysis of this Tröger's base analogue (as a racemic mixture)
confirms the structure. Very thin crystals (thickness of 0.01 mm) were obtained.
The structure found reveals a folding between the two acridine moieties. The
intramolecular dihedral angle between the least squares planes through the acridine
rings is 89.4o.
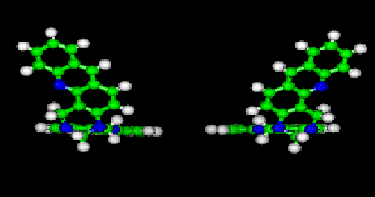
Tröger's bases are chiral molecules due to the blocked conformation of
the nitrogen atoms of the diazocine cycle (no inversion is possible). These
chiral molecules are characterized by a C2 axis of symetry. The two enantiomers are
presented here. One hypothesis of our research project is the chiral recognition
of DNA conformations. This hypothesis is supported by the first molecular
modelling study made by C. Coulombeau , C. Coulombeau and Y. Coppel in our laboratory.
Tröger's base analogue was partially resolved by crystallization of a
diastereomeric salt made with binaphthyl phosphoric acid. We obtained an enantiomeric
enrichment of 75%, and the circular dichroism spectra were taken.