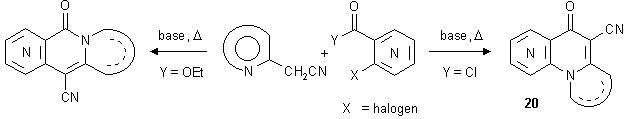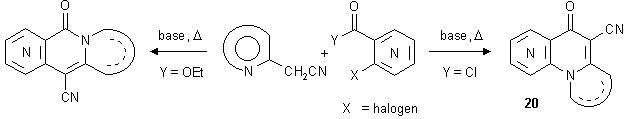
Synthesis of heterocycle-annelated naphthyridones with a bridgehead nitrogen atom
It is of interest that in all cases only linear annelated ring systems were obtained, although - in principle - the reaction might also take an alternative course: arylation of the ring nitrogen atom of the hetarylacetonitrile component and acylation of the activated methylene group would lead to structures of type 20 with nonlinear ring annelation. Compounds of the latter type (condensed [1,6]-, [1,7]-, and [1,8]-naphthyridines) have been isolated previously [13,14] when o-halogeno-substituted nicotinic or isonicotinic acid chlorides were employed as (more reactive) acylating agents.