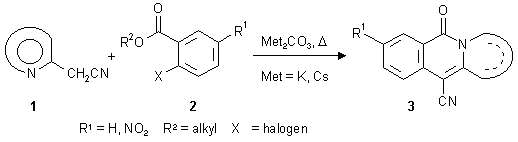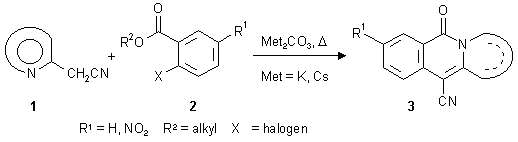
Synthesis of heterocycle-annelated naphthyridones with a bridgehead nitrogen atom
As shown recently, nucleophilic substitution reactions of hetarylacetonitriles of type 1 with 2-halobenzoates (2) in the presence of base give rise to the formation of fused isoquinolones of the linear structure 3 [1]. In continuation of previous investigations of this reaction type, we became interested in replacement of the benzoic-acid-type building blocks by N-heterocyclic analogues. By starting from heteroaromatic carboxylic acid derivatives like nicotinoates or isonicotinoates, respectively, with an appropriate leaving group (like a halogen) in the ortho position to the alkoxycarbonyl function, a variety of hitherto unknown hetarenonaphthyridones should become accessible. On the following pages, we describe the synthesis of a series of [b]-fused [2,7]naphthyridones (6-12), [g]-fused [1,6]naphthyridones (14-17), as well as a representative of a [b]-annelated [2,6]naphthyridone (19), using this methodology. Some of the new compounds (6-12, 16 and 19) are representative of hitherto unknown ring systems.