|
|
|
|
"There is something for these compounds that are being made, and they do, I think, have a very interesting future - both in the explosives area and potentially in the pharmaceutical area." -Philip P. Eaton.-
Although many physical properties of cubane have been measured, until about ten years ago cubane was considered just a laboratory curiosity of interest only to academics. Recently, however, the possible applications in industry, materials science and medicine have come to light. The fields of interest can be divided into the following categories:
First we shall consider the incredible explosive power of the cubane skeleton, and how this can be extended.
Explosives
Research on cubane explosives by Army and Army-contract scientists has sparked an unusual series of collaborations among military, academic, and industrial researches on the use of combinational chemistry and molecular modelling.
Cubane's potential as a source of high-energy explosives was first recognised in the early 1980s when Gilbert of the U.S. Army Armament and Development Command (now ARDEC) pointed out that the properties of cubane could make certain cubane derivatives important explosives.
The "effectiveness" of an explosive is dependent on many things. The energetics of the decomposition reaction and the number of moles and molecular weight of the gaseous products are among the critical factors. Density is also crucial. The more moles of an explosive that can be packed into the limited volume of a shell, the better. Less obvious, but more important, the velocity of the propagation wave through an explosive, i.e., the rate of energy release, is proportional to its density squared.
Cubane is a kinetically stable compound, but a thermodynamic powerhouse (see properties). It is one of the most dense hydrocarbons known (1.29 g cm-3) - higher that almost all other hydrocarbons (for comparison, the density of adamantane is approximately 1.09 g cm-3). As molecular volume is (very roughly) a group additive property, highly nitrated cubanes can be predicted to be very dense - and hence to be very powerful explosives.
Gilbert's colleagues, Sandus and Alster at ARDEC provided theoretical support and estimated that octanitrocubane might be 20 - 25% more effective than HMX (see box below for details), the present-day standard and an important explosive produced and used in large quantity. Octanitrocubane is shown below. It is easy to see that the detonation of octanitrocubane would lead to an awesome amount of very hot gas.
|
Octanitrocubane 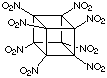 |
|
|
HMX 
|
HMX stands for High Melting eXplosive and is also known as octogen. Some information is given below: DHc = -660.7 kcal / mol DHf = 11.3 kcal / mol r = 1.89 g cm-3
|
The introduction of a few nitro groups, as we have already seen (see Reactivity), is straightforward in certain cases. For example the transformation of cubanecarboxylic acids to the corresponding amines via Curtius rearrangement, followed by oxidation. This method fails for cubanes with adjacent carboxylic acid groups. Although the corresponding vicinal diamines can be made and are relatively stable compounds, ring cleavage reactions intervene during the oxidation to a 1,2-dinitrocubane. This destroys the cubane system entirely. This methodology has been used to prepare 1,3,5-trinitrocubane and 1,3,5,7-tetranitrocubane, shown below:
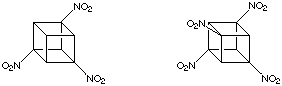
However, as all more highly nitrated cubanes necessarily have nitro groups on adjacent carbons none can be made without an alternative methodology. This is presented below.
The key to the problem was in the development of photochemical functionalization of cubanes, pioneered by A. Bashir-Hashemi and Jianchang Li at ARDEC in 1992. Fifty years ago, Kharasch and Brown reported the photochemical reaction of cyclohexane with oxalyl chloride to give cyclohexane carboxylic acid. This little known reaction has had limited synthetic application since rearrangements of the radical intermediates result in lower yields of the desired products. By virtue of the cubane geometry, the cubylradical (below) is an exception.
![]()
The cubane skeleton constrains the tertiary bridgehead radical to a pyramidal structure. Both hydrogen abstraction and b-sciccion in this species are unfavourable because these processes lead to intermediates of even higher energy, such as cubene, (shown below, left) and a radical species which is exceptionally strained because it is also a bridgehead alkene, (shown below, right).

In addition to this, the observation that cubane can be polyiodinated by means of radical reactions supports the view that photochemical carboxylation of the cubane skeleton could be synthetically useful.
Indeed, the reaction of commercially available 1,4-dimethoxoycarbonylcubane with oxalyl chloride, (COCl)2, under irradiation from a sunlamp for 6 hours produced, after esterification with MeOh, 1,2,4-trimethoxycarbonylcubane in 72% yield. The scheme is given below:
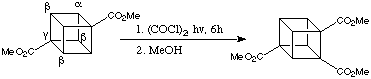
Extension of the reaction time to 48 hours led to the formation 1,2,4,7-tetramethoxycarbonylcubane and the pentacarbonylated derivative:

These reactions indicate a remarkable degree of regioselectivity which results from the reduced reactivity of the C-H bonds adjacent to the carbonyl groups. This therefore gives a simple and efficient photochemical procedure for the direct carboxylation of cubane has been demonstrated. In contrast to ortho -metalation, this methodology places the new functionalities at remote sites (b and g) and makes possible the synthesis of a wide range of cubanes.
The following scheme gives the conversion of the substituted methoxycarbonylcubanes to their nitrated analogues.
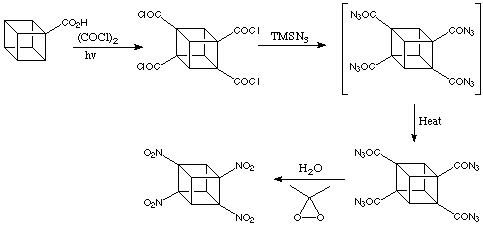
1,3,5,7-tetranitrocubane is a very powerful explosive, yet it is extremely stable, a great advantage from a safety standpoint. In addition, it has not exhibited toxicity in preliminary tests, meaning its use as an explosive probably would not necessitate environmental cleanup.
Octanitrocubane is the most powerful candidate explosive in the cubane class, but it has not yet been synthesised.
Recently, ARDEC and Geo-Centres researches were surprised to learn that cubane compounds may have biomedical applications as well. "Polynitrocubanes are superexplosives," says Sury Iyer, team leader for synthesis in ARDEC's energetics and warhead division, "but cubyl intermediates are also showing beneficial anti-viral, anti-AIDS, and anti-cancer properties.
Pharmaceuticals
Explosives and medicines are as different as night and day. One destroys life while the other attempts to save life. For example, a dipivaloylcubane -a cubane derivatized with keto, cyano, and amide groups, shown below- exhibits moderate activity against human immunodeficiency virus (HIV), which causes AIDS, without impairing healthy cells. In addition, a phenylcubane has shown moderate anti-cancer activity. An example of a dipivaloylcubane is shown below:
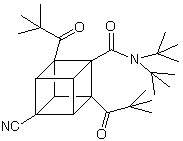
It has been found that alkylureas disrupt the lipid bilayer of the AIDS virus, and cubanes are highly lipophilic molecules that bind avidly to the envelope of the virus. It is possible that the use of these two types of compounds in tandem, or by linking them together, it may be possible to get a synergistic destructive effect on the virus envelope. Cubanes might also be useful as tiny antipathogen or anticancer bombs. The idea behind this concept is that cubanes could be bound to monoclonal antibodies and then deliver them specifically to pathogens or cancer cells in the body. Energy released from the high-energy cubanes would then be used to destroy the target.
Because the cubane frame is rigid, substituents have precise spatial arrangements to one another. The distance across the cube (the body diagonal) is almost the same as that between the para positions of a benzene ring. On cubane, one can add substituents in "the benzene plane", as well as above and below it, so to speak. This offers fascinating possibilities for the synthesis of new pharmaceuticals. When tetrachlorocarboxycubane is combined with a mixture of amino acids it is possible to synthesise a 12000 member combinational library of potentially active molecules. This is given below:
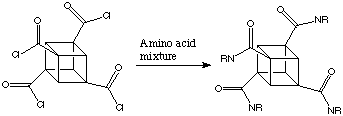
If the pentasubstituted cubane was used a library of more than a million variants could be created.
While some cubanes have been found to have some activity in anti-AIDS and anti-cancer applications, the activity / toxicity balance is not yet satisfactory. It is known, however, that the cubane system is not inherently toxic; most cubanes are biologically innocuous. The search for pharmaceutically significant cubanes has just begun. Presently, cubane should be viewed as a biologically stable, lipophilic platform on which the chemist can install a wide choice of substituents in a variety of well=defined spatial relationships. Developments in drug design programs should allow the judicious choice of substituents.
Polymers
Due to their well-defined dimensionality and rigid geometry, cubanes could prove to be important building blocks in the developing world of nanoarchitecture, in the form of oligomeric compounds. It is possible to synthesise oligomeric cubanes in the form of alkylated [n]cubylcubanes. This is shown in the following scheme:

One future application of this research is the possibility of synthesising liquid crystals with exceptional properties (e.g. UV transparency) from oligocubanes. To this end, materials such as the product shown below have been synthesised:
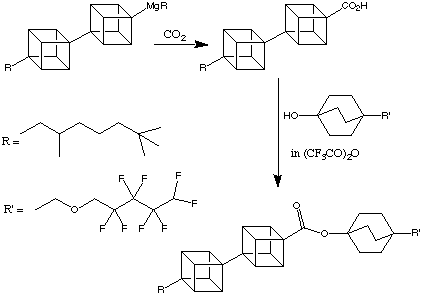
The phase transition temperatures are shown below:
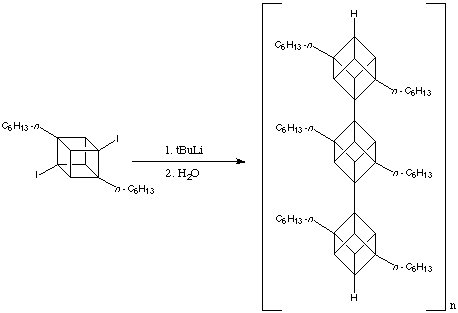
As the number of linked cubane units increases, that is, as n increases, the solubility of [n ]cubylcubanes plunges; at n = 3 most are essentially insoluble in ordinary solvents. This problem can be relieves by having moderately long alkyl substituents on the "monomer". Virtuani, a member of the Eaton research group has converted 2,7-di-n -hexyl-1,4-diiodocubane into the corresponding diyl, whose polymerisation, presumably initiated by addition of 4-iodo-1-lithio- or 2,7-di-n -hexyl-1,4-dilithio-cubane, gives an oligomeric cubylcubane with a molecular weight of approximately. This corresponds to a polycubyl rod about 150 Angstroms long containing on average 40 cubane units. The reaction is shown below:
|
|