Research Background
|
My PhD and postdoc. research were respectively with Dr.
Hamish Sutherland at Imperial College on the development of new peptide
coupling agents and with Prof. Alex Nickon at Johns Hopkins University,
Baltimore on photosensitised oxidation of steroidal olefins.
|

|
Key papers: J.Chem. Soc. 1964, 3495; ibid. 4650;
J.Am. Chem. Soc. 1965, 30, 1711.
|
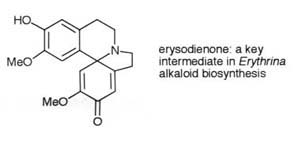
Further postdoctoral and initial faculty research was,
in collaboration with Prof Sir Derek Barton, Nobel Laureate, centred on
the structure determination, synthesis and biosynthesis of alkaloids, mainly
the Erythrina alkaloids, and the fungal steroids of the yeast Saccharomyces
cerevisiae.
|
|
Key papers: Abh. der Deut. Akad. der Wiss., Berlin
1971, 7; J. Chem. Soc., Perkin Trans. I, 1974, 1326.
|
Concurrent with the above work, I initiated studies on
models for the biochemical mechanisms of NADH and coenzyme B12 dependent
reactions. In particular, the free radical migration of the thiol ester
in methylmalonylCoA mutase models was demonstrated and, in collaboration
with
Dr. Henry Rzepa,
an order of migratory aptitude for acyl residues, under radical conditions,
predicted.
|

|
Key papers: J. Chem. Soc., Chem. Commun., 1983,
625; J. Chem. Soc., Perkin Trans. I, 1986, 1139.
|
From biosynthesis to biotransformations is a the logical
step for an organic chemist and recent bio-work, in collaboration firstly
with Professor Doug Ribbons and more recently with
Dr. David Leak,
has been on the application of dihydroxylase, oxidase
and hydratase enzymes, either in whole cells or in purified form, to organic
synthesis.
|

|
Key papers: J. Chem. Soc., Perkin Trans. I, 1995,
2647; Tetrahedron Lett., 1996, 37, 8819
|
A long standing collaboration with Professor Vic Pike
of the MRC Cyclotron unit at Hammersmith Hospital involves a search for
synthetic methods compatible with 18F technology for application in Positron
Emission Tomography (PET). This has resulted in the development of fluorodestannylation,
fluorodeboronation and fluorodeindiation by caesium fluoroxysulfate and
the PET compatible synthesis of fluorosteroids and other pharmaceutical
agents.
|
|
Key papers: Tetrahedron, 1992, 48, 8073;
J. Chem. Soc., Perkin Trans. I, 1995, 2965.
|
In the late seventies, I made a major move into the application
of chemo- , regio-, and stereo-specific transition metal mediated reactions
to the synthesis of bioactive molecules. This resulted in the discovery
of new ortho - directing effects in arenetricarbonylchromium(0)
complexes and to the development of new methods for the remote functionalisation
of the aromatic ring in these complexes.
|
|
Key papers: Tetrahedron Lett., 1986, 27, 5525;
Phil. Trans. R. Soc. Lond., 1988, A 326, 595.
|
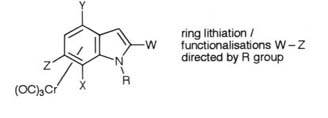
Heterocyclic arenetricarbonylchromium(0) complexes proved
to be an equally fertile area of novel chemistry and the principles of
remote functionalisation were applied to the indole complexes and resulted
in the development of methods for functionalisation at positions 4-, 6-,
and 7- on the indole ring.
|
|
Key papers: Tetrahedron, 1988, 44, 7325;Tetrahedron,
1989, 45, 5955.
|
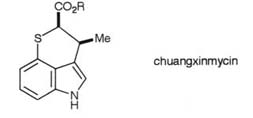
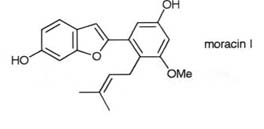
These techniques were exemplified in the synthesis of chuangxinmycin,
a broad spectrum antibiotic found in the chinese soil bacterium Actinoplanes
tsinanensis and in the synthesis of the moracins and related resorcinylbenzofuran
antifungal agents from the white mulberry tree, Morus alba.
|
 |
|
|
Key papers: J. Chem. Soc., Perkin Trans. I, 1992,323;
Tetrahedron, 1991, 47, 8621.
Last updated: 11th November 2001






