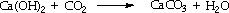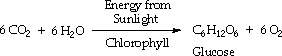
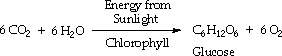
Glucose and other simple carbohydrates can be converted to more complicated carbohydrates such as starch and cellulose.
Carbon dioxide is the fourth most abundant gas in the atmosphere, but on the planets Venus and Mars it is the most abundant. The gas traps infrared radiation emitted from the warm earth surface. Carbon dioxide is transparent to sunlight, the light warms the surface, as infrared radiation is emmited back, it is trapped by the molecules and the atmosphere is warmed in a process known as the greenhouse effect.
In the laboratory, the test for carbon dioxide is to bubble it through dilute calcium hydroxide solution (limewater). This results in the formation of insoluble calcium carbonate which makes the solution turn cloudy.