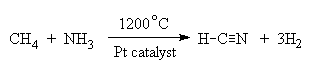
Hydrogen cyanide, HCN, is a colourless gas that smells slightly of almonds (although a small proportion of people, about 1 in 10, cannot smell it at all due to a genetic trait). It is extremely poisonous, because it binds irreversibly to the iron atom in hemoglobin, making it unavailable to transport the vital O2 to the body's cells and tissues. It also interferes with ATP (adenosine triphosphate) the main energy storage molecule in the body. The combination of both these two events rapidly bring the body's metabolism to a halt, resulting in death.
Ironically, for such a lethal molecule, it is thought to have been one of the main progenitors of life on Earth. This is because HCN was probably one of the small molecules in the Earth's primeval atmosphere and would therefore have been an important source or intermediate in the formation of biologically important chemicals. For example, under pressure and with traces of water and ammonia, HCN produces adenine, one of the bases needed to construct DNA. HCN can also act as a condensing agent to turn amino acids into the polypeptides from which complex proteins are made.
HCN is evolved when metal cyanides such as NaCN or KCN are treated with acids. This is one method by which prisoners in US jails are executed - they are strapped into a chair in a sealed, airtight room, and concentrated acid is dropped onto a large amount of a metal cyanide salt...
HCN has a number of innocuous uses as well. It is made commercially on a large scale by the reaction: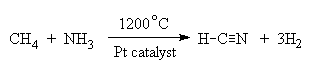
from which it is used to make adiponitrile (NC(CH2)4CN), which is then used to produce nylon. Waste HCN is converted for use as a fertiliser.
Cyanide is commonly thought of as a gas, but you also can be poisoned by it if you ingest wild cherry syrup, prussic acid, bitter almond oil, or large amounts of apricot pits. Cherry seeds, peach and plum pits, corn, chickpeas, cashews, and some other fruits and vegetables contain cyanogenic (i.e., cyanide-forming) glycosides (such as amygdalin) that release hydrogen cyanide when chewed or digested. As a result, some cyanide can also be found in fruit jams that contain these pit and pip extracts, such as quince. However, since the concentration of cyanide in these compounds is small, accidental cyanide poisoning from a food source is rare. But, if the correct materials are deliberately concentrated it can make an effective poison, as the Romans and Egyptians knew. They used to grind up peach kernels to make poisons.
The first symptoms of cyanide poisoning are rapid heartbeat, headache, and drowsiness - followed by coma, convulsions, and death. At this point the victim's face is usually bright red as a result of the change in hemoglobin mentioned above. Since cyanide poisoning usually kills people in less than 15 minutes, immediate treatment is absolutely essential. However, cyanide poisoning is not automatically lethal. It is possible to survive mild doses of cyanide. The way to treat cyanide poisoning is to undo the HCN-hemoglobin binding process, and administer 100% oxygen to support respiration. First induce vomiting if possible, or wash the stomach with a saline solution. Then give, of all things, amyl nitrite, since this is a specific antidote. Alternatively, you could give an intravenous infusion of sodium nitrite, followed by an infusion of sodium thiosulphate. Both these chemicals are contained in a standard 'cyanide antidote kit' which is required by any workers using cyanide compounds in industry or universities.