 | 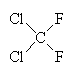 | 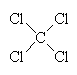 | 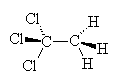 |
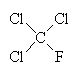
| CFC-11 | CFC-12 | Carbon tetrachloride | 1,1,1-trichloroethane |
The chlorofluorocarbons (or CFCs for short) have recently received a great deal of attention, and notoriety, as a result of being implicated in the destruction of the ozone layer. CFCs (also known as Freons) are a family of chemicals based upon hydrocarbon skeletons (most often methane), where some or all of the hydrogens have been replaced with chlorine and/or fluorine atoms. These compounds are non-flammable, tasteless and odourless, and chemically stable. Their other important property is their volatility, having boiling points close to zero degrees Centigrade. These physical properties make them ideal for use as refrigerant gases in air conditioners, freezers and refrigerators. Their low boiling points also make them ideal for blowing agents for foam plastics, allowing the foam to expand as the liquid CFC boils.
 |  |  |  |
| CFC-11 | CFC-12 | Carbon tetrachloride | 1,1,1-trichloroethane |
An example of a refrigerant CFC is dichlorodifluoromethane, CF2Cl2 (also known as CFC-12), which boils at -30°C. Another once-common CFC is trichlorofluoromethane, CFCl3 (CFC-11), which boils at 24°C and was once the propellant in around half of all the aerosol cans used in the world. These and other CFCs, and the chemically similar methylene chloride, CH2Cl2 and carbon tetrachloride, CCl4) were often used as solvents for cleaning computer parts, printed circuit boards, and as 'dry cleaning' agents for clothes. Carbon tetrachloride itself was once used as a fluid in certain fire extinguishers. Another well-known chlorocarbon solvent is 1,1,1-trichloroethane, which was once used in typing correction fluid (under the name 'Tippex').
 It is ironic that one of the properties that make CFCs so suitable for use in household items such as refrigerators, their chemical stability, should be the cause of their undoing. Most chemicals, when released into the atmosphere get rapidly broken down into smaller, harmless components by reactions in the lower atmosphere. The CFCs however, are so stable and unreactive that they survive to reach the highest levels of the atmosphere, and become globally distributed in the stratosphere. At these high altitudes, the intensity of ultra-violet radiation is so great that even the stable CFCs are split apart to release a chlorine atom.
It is ironic that one of the properties that make CFCs so suitable for use in household items such as refrigerators, their chemical stability, should be the cause of their undoing. Most chemicals, when released into the atmosphere get rapidly broken down into smaller, harmless components by reactions in the lower atmosphere. The CFCs however, are so stable and unreactive that they survive to reach the highest levels of the atmosphere, and become globally distributed in the stratosphere. At these high altitudes, the intensity of ultra-violet radiation is so great that even the stable CFCs are split apart to release a chlorine atom.
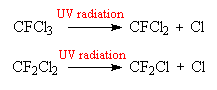
It is the atomic chlorine that does the damage, since it can react with ozone (O3) to form oxygen.
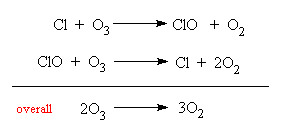
The Cl atom is regenerated in this reaction, and so the breakup of only one CFC molecule can initiate the subsequent removal of thousands of ozone molecules. This has recently become a major cause for concern, since the ozone forms a vital protective layer in the upper atmosphere, shielding the Earth and all its plants and animals from the harmful effect of the Sun's UV radiation. Damage to the ozone layer would let more UV through to reach the planet's surface, leading to an increase in skin cancers in animals and humans, and damage to vegetation. The discovery of the 'hole' in the ozone layer above the Antarctic in the 1980s, led to a United Nations protocol to reduce the use of CFCs and other chlorocarbons as solvents, refrigerants and aerosols by 50% by 1999, and a total ban soon after. CFCs are now banned from aerosols, and replacements for their use in refrigeration (based on ammonia or the less dangerous hydrofluorocarbons, HFCs) are now being introduced. However CFCs are so tenacious, that it will take many years before they are all removed from the atmosphere. Some estimates say that the ozone layer may not re-establish itself properly for a century or more.