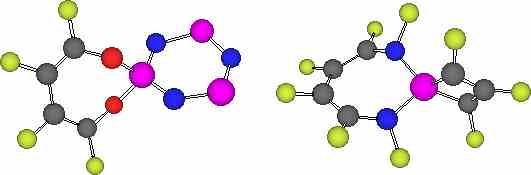
Summary: We propose spiroaromatic ring systems
characterised by having a common coarctate phosphorus atom in
which each ring can independently exhibit Möbius or
Hückel aromaticity.

| Dioxaphosphepine examples | ||
|---|---|---|
| bobwoe | bobwiy | bobweu |
| Dioxaphosphepine examples | ||
| JOWFAC | LODHOB | OXPCTP10 |
| Diazaphosphepine example | ||
| WEMTAJ | ||
| Dioxaphosphole and Diazaphosphole example | ||
| DOFSUM | YUJFOV | |
| Triarsenoazobenzene example | ||
| HPTARZ | ||
| Calculated energies (Hartree) and NICS Values (ppm) for 1-6 | |||||
|---|---|---|---|---|---|
| Substituents | Energy | NICSa | Substituents | Energy | NICSa |
| 1, X=O, R=H | -1493.59245 | 1.0 (-2.2)/-0.4 | 1, X=NH, R=H | -1453.84743 |
1.9 (-1.8)/-0.3 |
| 1, X=O, R=benzo | -1800.91060 | 1.5 (-1.7)/1.2 | |||
| 1, X=O, R=F | -1890.49767b | 0.9 (-2.3)/-5.0 | 1, X=NF, R=F | -2049.05166 (-2049.04717)c |
1.5 (-2.0)/-9.6 |
| 2, X=O, R=F | -1237.02783b | -7.9 (-7.7)/-5.9 | 2, X=N, R=F | -1395.58808 (-1395.57697)c |
-7.7(-7.6)/-11.6 |
| 2, X=S, R=F | -1882.94836 | -5.3/-9.5 | |||
| 2, X=O, R=F, Arsenic | -3129.40995 | -7.6 (-8.0)/-7.8 | 2, X=NF, R=F Arsenic | -3287.97290 | -7.3 (-7.8)/-12.2 |
| 3, X=O, R=F | -1614.62798 |
0.3/-7.8 | 3, X=NF, R=F |
-1773.19255 (-1773.18420)d |
2.4/-9.2 |
| 4, X=O, R=F | -961.16074 |
-8.8 (-8.6)/-7.1 | 4, X=NF, R=F |
-1119.72821 (-1119.72181)d |
-7.7 (-7.5)/-9.2 |
| 5, X=O, R=F | -1494.32476 |
-1.2/-4.5 | 5, X=NF, R=F |
-1652.88226 |
0.4/-8.6 |
| 6, X=O, R=F, R'=H | -1159.51846 |
2.6/-4.8 |
6, X=NF, R=F, R'=H |
-1318.07670 |
5.2/-8.3 |
| 6, X=O, R=F, R'=F | -1457.18499 | -17.2/-4.6 | 6, X=NF, R=F, R'=F |
1615.74390 (-1615.74177)e |
-16.2/-8.3 |
aNICS0(NICS1) values at respectively the ring centroid and 1Å above ring centroid, for respectively the phosphabenzene or cyclophosphazene and the diheterophosphepine ring.bGeometry optimisation converges to C2 symmetric conformation. cCs conformation. dAnti-conformation, with no symmetry. eChiral diastereoisomer.