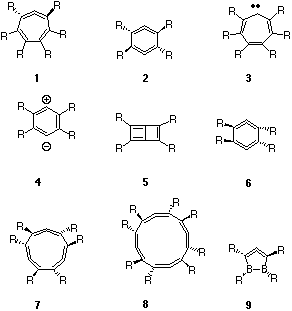
Both 1 and 2 can have planar 4n+2 p-aromatic valence bond isomers 3 and 4 (p-benzyne)5 respectively, although these may not be stable minima but can also be transition states for chiral inversion. Here we present an analysis of the key features of the Möbius annulenes in terms of p-molecular orbital correlation between the planar Hückel (H) aromatic stationary point and the twisted C2 symmetric Möbius (M) system. We recognise that the s framework also has Möbius features, but do not deal with those in the present article.
Table 1. Calculated Energies for 1-9.
|
System |
AM1 (kcal/mol) |
B3LYP/6-31G*,Hartree |
NICS(0),ppm |
|---|---|---|---|
|
1, R=H
|
|||
|
3, R=H
|
|||
|
1, R=F
|
|||
|
3, R=F
|
|||
|
2, R=H
|
-230.7879d
|
||
|
2, R=t-Bu
|
-
|
-
|
|
|
4, R=H
|
|||
|
4, R=t-Bu
|
-
|
-
|
|
|
2, R=F
|
|||
|
4, R=F
|
13.7c
|
-627.6986e
|
|
|
5, R=t-Bu
|
-
|
-
|
|
|
5, R=F
|
|||
|
6, R=F
|
|||
|
7, R=F
|
|||
|
8, R=F
|
|||
|
9, R=F
|
|||
| a Transition state, with one imaginary normal mode; 301.5i cm-1. b Transition state, with one imaginary normal mode; 274.6i cm-1. c Transition state, with one imaginary normal mode; 1093.7i cm-1. d AM1 geometry for 2. e. AM1 geometry for 4. f For D2 syummetry. Value for D4 symmetry in parentheses. | |||
The symmetric 1b1 p-orbital of 3 correlates with 5b from 1, the latter being best described as a perturbed H-type orbital. Similarly the 2b1 p-orbital correlates with the H-type orbital 8b. The third occupied 1a2 p-orbital (corresponding to what would be a degenerate partner of 2b1 in e.g. benzene) correlates with the C2 symmetric 8a orbital in 1, and this latter orbital is a clear example of a true Möbius (M) orbital, having no node in the plane of the molecule and hence presenting a single p-face for the system. The original carbene-like orbital in 3 (8a1) similarly transforms to a C2 symmetric 9a M-type orbital in 1. Both 8a and 9a can be regarded as the key orbitals which impart p-Möbius characteristics to the system. One unusual aspect of these orbitals is their nodal properties. The 9a orbital has one phase forming two rings in a twisted figure eight shape, the other phase passing continuously through the each of the two rings (this can be seen more clearly when the orbitals are contoured at a lower level). The lower energy orbital 8a has one phase forming a twisted S shape, with the other phase passing through each of the two arcs. Heilbronner1 noted that a formal HMO solution of the secular equations including a distributed Möbius overlap (resonance integral) results in a degenerate HOMO. Although the C2 symmetric 8a and 9a differ by 1.6 eV (AM1) in energy, at least part of their energy separation is due to interactions with the s framework, not considered in the Heilbronner argument. More significant are the chiral properties of these two Möbius orbitals. Thus the direction of "twist" of 8a superimposed on the chirality defined by the nuclear positions is the opposite of 9a. Such chiral properties may well be a unique and characteristic feature of such pairs of Möbius orbitals.
The one remaining occupied p-orbital correlation is more ambiguous. A simple analysis indicates that three p-Hückel and one carbene-like s orbital in 3 should correlate with four occupied p-Hückel and p-Möbius orbitals in 1. However, the physical form of 7b suggests that it resembles the nodeless p orbital 5b, but differs in having two s-plane nodes along the C-H bonds. For orbitals such as this one, the s/p distinction may be difficult to make. Overall, 1 can be regarded as either a 2H + 2M, or as a 3H + 2M system if the 7b is regarded as having substantive p character. The computed NICS(0) value (B3LYP/6-31G*) for 1 is -5.7 ppm, indicating it to have only modestly diatropic ring currents compared with e.g. benzene (-9 ppm including paratropic contributions of the s-bonds, or -21 ppm for the p-only contribution).
The virtual orbital correlations indicate that 10a and 11a are both Möbius, and 9b and 10b are Hückel, although the latter does appear to have features which indicate nodeless connectivity between the top and bottom surfaces of the molecular plane.
The two H-type orbitals in 1 (R=F) are 16b and 17b, and the two M-type orbitals are 17a and 18a. These are closer to energy degeneracy than was the case with R=H, due we think to the reduced mixing with the s system, 18a having clearly much less C-R character than did 9a (R=H). This orbital also appears to have some of the in-plane p-conjugation noted by Schleyer and co-workers for the trannulene series.7. The two M-type orbitals again have opposite chirality. Another descriptor ithat could be applied to the two M-type orbitals is that 18a has rotaxane features, whilst 17a has catenane features. The distinction between these two features appears at least in part to be determined by the nature of the C-R bonds. It is also noteworthy that the rotaxane can be considered as having one more node than the catenane.
The NICS value 1 (R=F) of -13.2 ppm indicates perfluorination significantly increases the overall NICS(0) value of both the Möbius and the planar Hückel aromatics. A dissection of the individual orbital contributions in 1 to identify the precise origins of this effect is clearly a future requirement. In particular it would be important to establish whether the effect is associated with the elimination of the psuedo p orbital (7b for R=H) or with the reduced energy gap between the top two occupied orbitals.
We also investigated the relative energies for the bulky substituent R=tBu, arguing that steric repulsion between the two bulky groups would be minimised in 2 and hence might stabilise it. This isomer is indeed calculated (AM1) as 8.5 kcal mol-1 lower than singlet 4. However, as a synthetic target, 2 is unlikely to be viable, since the triplet state is calculated to be lower in energy than any of the singlets.
The orbital correlation diagrams (Figure 3) show many similarities with 1/3. For AM1-derived 2, R=H, two H-type orbitals are identifiable as 2b3 and 4b2, two M-type orbitals 3b1 and 4a, and again an ambiguous orbital (3b3) which derives from the s framework but has distinct p features. The relative orbital energies for 1 and 2 are also similar.
The two non-degenerate Möbius orbitals 3b1 and 4a in 2 (R=H) have similar forms to those of 1, R=H. Strikingly, the Möbius LUMO (4b1) has the same nodal properties as the HOMO but has opposite chirality, which as noted above may be a characteristic feature of Möbius systems. An approximate analogy with conventional stereochemical notation is that 3b1 and 4b1 form chiral diastereoisomers with respect to the nuclear framework, one bonding, the other antibonding. Again, we note that the 3b1 and 4b1 orbitals resemble a catenane, whilst the 4a orbital resembles a rotaxane. Finally, we note that in group theoretical terms, the 3b1 orbital in 2 correlates with the 8a Möbius orbital in 1 when the common C2 symmetry element is considered.
Both 2, R=H and F are predicted to have strongly paratropic ring currents (Table), which contrasts with the diatropic characteristics for 1. The nodal properties, energies and energy separations of the two Möbius HOMO p-like orbitals in 2, R=F (6b1 and 7a) appear to be very similar to their the equivalents in 1, R=F (17a and 18a) The ring current corresponds to a single-electron exitation operator, which would be expected to originate predominantly in the two symmetry allowed HOMO-LUMO transitions arising from the 6b1 to 7b1 or the 7a to 8a excitation in 2. The other transitions, e.g. 7a to 7b1 are symmetry forbidden. In contrast, the active space of four Möbius orbitals of a symmetry in 1 would give rise to four possible allowed excitations. To establish whether this effect might contribute to the paratropic character of 2, we also computed the C2 symmetric 1,3-difluoro substituted system. The NICS value for this was +20.3 ppm, similar to +22 - +23 for R=H or F. This suggests that another factor must be responsible for the differing behaviour of 1 and 2. The correlation of ground state 2 with doubly excited (and anti-aromatic) 4 may indicate that the nature of 2 is associated with the orbital occupancy and not the nodal characteristics of the Möbius orbitals. To ascertain whether ring current was associated specifically with the relative chirality and hence phases of the two C=C=C units, we next studied the isomer 6.
Along the series n=1-4, equal numbers of occupied M- and H-like orbitals are formed (Figure 5). The computed NICS values (Table) indicate aromaticity for n=1 and 3, anti-aromaticity for n= 2, 4, although attenuating as the ring size increases. This in fact corresponds to a 4n+2 rule for aromaticity as applied to the total number of either Möbius OR Hückel orbitals! The single Möbius HOMO orbital for 9 and the doubly degenerate Möbius HOMO orbital for 7 also follow the pattern found for 2-electron and 6-electron Hückel aromatics. The anti-aromatic 2 and 8 may not have degenerate HOMO orbitals, possibly because, like cyclobutadiene, of a contribution from Jahn-Teller distortion. This latter conclusion is supported by the distortion of 8 from the expected D4 symmetry to the lower energy D2 form. Along this series at least then, one can conclude that electron counting rules must be applied individually to the Möbius and Hückel occupied orbitals, rather than jointly. Doing this, one arrives at a 4n+2 rule for aromaticity in this series. In effect, if each C=C=C unit is regarded as contributing one phase inversion, then aromaticity arises from an odd number of such inversions, and anti-aromaticity (or Jahn-Teller-like distorsion) from an even number.
In system 1 which involves the interaction of a 4-p C=C=C unit with 4 p electrons in a diene unit, the result is a pair p occupied Möbius-like and a pair of Hückel-like orbitals. 4n Electron occupancy of the two lowest energy Möbius orbitals then gives rise to overall aromaticity. For the series of which 1 is a member, additivity of chirality of the C=C=C units results in a series where 4n+2 occupied Möbius orbitals results in aromaticity and 4n occupancy results in anti-aromaticity.
A further generalisation for how one or more C=C=C units interact with alkenes and alkynes in cyclic annulene-like systems is clearly a desirable future objective, along with an analysis of how higher order cummulenes (e.g C=C=C=C=C) can interact to produce Möbius-like molecules.
Acknowledgements: We thank P. v. R. Schleyer and M. Mauksch for stimulating discussions.