 | Nitroglycerin - is derived from the glycerol molecule (highlighted in red) which is a common biological molecule from which triglycerides fats and oils are constructed, where all the -OH groups have been replaced by -NO2. |
Nitroglycerin is an oily, colourless liquid, but also a high explosive that is so unstable that the slightest jolt, impact or friction can cause it to spontaneously detonate. Since the molecule contains oxygen, nitrogen and carbon, when it explodes a lot of energy is released as the atoms rearrange to form new molecules with strong, stable bonds, like N2 and CO.
 | Nitroglycerin - is derived from the glycerol molecule (highlighted in red) which is a common biological molecule from which triglycerides fats and oils are constructed, where all the -OH groups have been replaced by -NO2. |
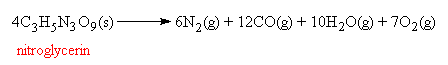
It is the speed of the decomposition reaction which makes nitroglycerin such a violent explosive. Unlike burning, which can only travel as fast as the flame front can move through the material, high explosives are decomposed almost instantaneously by a supersonic shock wave passing through the material. This instantaneous destruction of all the molecules in the sample is called a detonation, and the rapid expansion of hot gases that results is what causes the destructive blast. In fact, 4 moles of nitroglycerin produces 35 moles of hot gases. One advantage that nitroglycerin has over some other high explosives, like TNT, is that no solid forms of carbon (in the form of soot or smoke) is produced when it is detonated. This allows nitroglycerin to be used to make 'smokeless powders', which is of great advantage to artillery or naval gunners whose field of vision does not then become obscured during battle by clouds of billowing smoke.
Nitroglycerin has one major disadvantage, however - it is very, very unstable. To be a useful explosive, a substance has to be able to withstand, without detonating, the jolts and bumps both of its manufacture, and of its transportation to where it will be used. Clearly, nitroglycerin is far too dangerous for this, and many people lost their lives in the last century trying to use nitroglycerin for peaceful purposes (like quarrying). The man that solved this problem, was the Swedish chemist Alfred Nobel.
 | Alfred Nobel - the discoverer of dynamite |
 For several years Nobel had been working in Stockholm on developing nitroglycerine as an explosive. He manufactured it by (carefully) mixing glycerol with nitric and sulphuric acids. But several explosions in his laboratory, including one in 1864 in which his brother Emil and several other persons were killed, convinced the authorities that nitroglycerine production was exceedingly dangerous. They forbade further experimentation with nitroglycerine within the Stockholm city limits and Nobel had to move his experimentation to a barge anchored on Lake Mälaren. Alfred was not discouraged and in 1864 he was able to start mass production of nitroglycerine. To make the handling of nitroglycerine safer, Nobel experimented with different additives. He soon found that mixing nitroglycerine with a type of clay called kieselguhr, would turn the liquid into a paste which could be shaped into rods of a size and form suitable for insertion into drilling holes. In 1867 he patented this material under the name of dynamite. Nowadays, though, the standard dynamite used in the US is composed of nitroglycerin, ammonium nitrate and sodium nitrate (2 other explosives), wood pulp (the absorbing medium), and a trace of calcium carbonate to neutralise traces of acids that might form during storage.
For several years Nobel had been working in Stockholm on developing nitroglycerine as an explosive. He manufactured it by (carefully) mixing glycerol with nitric and sulphuric acids. But several explosions in his laboratory, including one in 1864 in which his brother Emil and several other persons were killed, convinced the authorities that nitroglycerine production was exceedingly dangerous. They forbade further experimentation with nitroglycerine within the Stockholm city limits and Nobel had to move his experimentation to a barge anchored on Lake Mälaren. Alfred was not discouraged and in 1864 he was able to start mass production of nitroglycerine. To make the handling of nitroglycerine safer, Nobel experimented with different additives. He soon found that mixing nitroglycerine with a type of clay called kieselguhr, would turn the liquid into a paste which could be shaped into rods of a size and form suitable for insertion into drilling holes. In 1867 he patented this material under the name of dynamite. Nowadays, though, the standard dynamite used in the US is composed of nitroglycerin, ammonium nitrate and sodium nitrate (2 other explosives), wood pulp (the absorbing medium), and a trace of calcium carbonate to neutralise traces of acids that might form during storage.

 With the invention of dynamite, there was at last a way of utilising the destructive power of the explosive in a relatively safe form. The new safe explosive, dynamite, immediately found many industrial uses, such as mining, quarrying, demolition, etc, but then the first World War began and Nobel's discovery was used to fuel the war machines on both sides. Despite being a pacifist, Nobel continued to produce nitroglycerin-based explosives, justifying this apparent anomaly by saying he was trying to produce weapons so powerfully destructive that no-one would dare to use them - thus eliminating war. However, the mass destruction of WW1 and later wars showed that this strategy didn't work. Soon after WW1 a newspaper mistakenly published Nobel's obituary, before he had actually died. Nobel was horrified to read that he would be remembered for eternity as the man who created the explosives that caused so much death and carnage. Maybe to partially alleviate his conscience, and to make his name more famous for a better reason, he used his great fortune to instigate the Nobel prizes for achievements in science, medicine and peace.
With the invention of dynamite, there was at last a way of utilising the destructive power of the explosive in a relatively safe form. The new safe explosive, dynamite, immediately found many industrial uses, such as mining, quarrying, demolition, etc, but then the first World War began and Nobel's discovery was used to fuel the war machines on both sides. Despite being a pacifist, Nobel continued to produce nitroglycerin-based explosives, justifying this apparent anomaly by saying he was trying to produce weapons so powerfully destructive that no-one would dare to use them - thus eliminating war. However, the mass destruction of WW1 and later wars showed that this strategy didn't work. Soon after WW1 a newspaper mistakenly published Nobel's obituary, before he had actually died. Nobel was horrified to read that he would be remembered for eternity as the man who created the explosives that caused so much death and carnage. Maybe to partially alleviate his conscience, and to make his name more famous for a better reason, he used his great fortune to instigate the Nobel prizes for achievements in science, medicine and peace.