Nitrogen Oxides and Nitric Acid
NOXious Gases
 The oxides of nitrogen, nitric oxide, NO, and nitrogen dioxide, NO2, are both collectively known as NOx, and are familiar as one of the several components of automobile exhaust that create smog. In the hot combustion chambers of a car's engine, N2 and O2 react together to form NO. This process is normally unfavourable, but at high temperatures in the combustion chamber the reaction proceeds rapidly. The NO passes into the exhaust with the other gases, and then cools so rapidly that the NO can't revert back into nitrogen and oxygen again, because the reaction rate becomes too slow at these lower temperatures. Once released into the atmosphere, NO reacts to form NO2, which is familiar as the brown hazy colour of smog which is often seen clinging above modern cities. The problem gets worse, however, since NO2 can then be split apart by ultra-violet radiation from the sun, to produce oxygen atoms. These then react with oxygen molecules to make ozone. On calm days during summer, this ozone can build up close to the ground in cities, causing numerous respiratory problems among pedestrians.
The oxides of nitrogen, nitric oxide, NO, and nitrogen dioxide, NO2, are both collectively known as NOx, and are familiar as one of the several components of automobile exhaust that create smog. In the hot combustion chambers of a car's engine, N2 and O2 react together to form NO. This process is normally unfavourable, but at high temperatures in the combustion chamber the reaction proceeds rapidly. The NO passes into the exhaust with the other gases, and then cools so rapidly that the NO can't revert back into nitrogen and oxygen again, because the reaction rate becomes too slow at these lower temperatures. Once released into the atmosphere, NO reacts to form NO2, which is familiar as the brown hazy colour of smog which is often seen clinging above modern cities. The problem gets worse, however, since NO2 can then be split apart by ultra-violet radiation from the sun, to produce oxygen atoms. These then react with oxygen molecules to make ozone. On calm days during summer, this ozone can build up close to the ground in cities, causing numerous respiratory problems among pedestrians.
Cats and Acid Rain
To combat the problems of smog, cars are increasingly being fitted with catalytic converters ('cats'), which mix air with the exhaust gases, over an appropriate catalyst (usually a rare metal like platinum or palladium) and promote oxidation of unburnt fuel to CO2. They also cause the decomposition of nitrogen oxides back to harmless N2 and O2. Another method for controlling NOx emissions involves mixing water with the air-fuel mixture. It works because some of the heat from the combustion is absorbed by the water vapour, so the mixture of exhaust gases doesn't get so hot, and at these lower temperatures, the concentration of NOx gases is much reduced.
The problem of NOx gases doesn't disappear on cloudy days, however. In this case, the NO2 survives to reach higher altitudes, and, (along with SO2), dissolves in the water droplets in clouds to give acids, such as nitric acid (HNO3), and nitrous acid (HNO2). This corrosive mixture can then fall back to Earth as 'acid rain', which can be as acidic as lemon juice (pH 2.2-2.7). In fact, the highest recorded acidity from acid rain was as acidic as vinegar, and occurred at Pitlochry, Scotland, in April of 1974. Acid rain is a major environmental problem, causing devastation of forests and lakes, and corrosion of buildings and statues.
 | A spruce forest damaged and dying as a result of Acid Rain
|
The nitrogen oxides are not all bad. They are both used in the lead chamber process as catalysts to convert SO2 to SO3, which is the essential first step in the production of sulphuric acid. They are also precursors in the Ostwald Process for the manufacture of nitric acid (see below).
NO2 - a Strange Dimer
NO2 is an odd molecule since it has an unpaired electron sitting on the nitrogen. This makes it paramagnetic, and also allows it to react with itself to form the dimer, dinitrogen tetroxide, N2O4. But this dimer bond is quite weak, and as the temperature is raised it rapidly dissociates back to NO2.
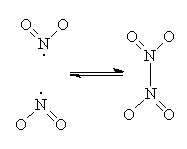
The position of the equilibrium between the two compounds and the colour of the system vary with temperature. Below -21°C, only pure, solid N2O4 is present, which is colourless. At -21°C, the system melts, and even though there is only 0.01% NO2 present at this temperature, it is enough to make the system pale yellow. The mixture boils at 21°C and at this temperature contains 0.1% NO2, making it a deep-reddish-brown colour. Above 140°C the system is 100% NO2 and an even darker reddish brown. Under the correct circumstances, N2O4 can be an oxidising agent, and indeed has been used as an oxidant in space shuttle engines.
Nitric Acid
Nitric acid (HNO3) is an extremely important chemical used in the manufacture of fertilisers and explosives. It is made from ammonia by the Ostwald Process (developed in 1902 by the German chemist Wilhelm Ostwald, who got the Nobel prize in 1909). This process reacts together O2 and NH3 at 850°C and 5 atmospheres pressure, with the help of Platinum and Rhodium catalysts, to make NO. This is then oxidised to NO2, which is then dissolved in water to make HNO3. The Ostwald process was discovered just in time for the First World War, and it contributed greatly to the extended length of that war. This is because previously Germany had no nitrate deposits of its own from which to make the nitric acid that was essential for the production of the explosives used in artillery shells, such as TNT and nitroglycerin. In fact, most of the nitrates were only available from guano, which is the droppings of fish-eating sea birds, and is found in large quantities on the islands off the coast of Peru. When hostilities began, the shipping routes to Germany across the Atlantic were blocked, and so the Ostwald process gave Germany the ability to carry on the war far longer than it would otherwise have been able.
 | Nitric acid is used to make high explosives such as TNT
|
100% pure, anhydrous nitric acid is a colourless anhydrous solid. What we call 'concentrated nitric acid' is actually a solution of 68% by weight HNO3 in water (16M), and is often pale yellow as a result of photochemical decomposition which gives NO2. By dissolving even more NO2 into the pure material produces red 'fuming' nitric acid, which is an extremely powerful acid and oxidising agent using in the semiconductor industry for cleaning silicon wafers. Aqua Regia (approx 3 vols HCl to 1 vol HNO3) contains free Cl2 and nitrosyl chloride (NOCl). This powerful acid attacks even the inert metals gold and platinum owing to the ability of Cl- to stabilise the complexes AuCl4- and PtCl62-.
An Explosive Beginning
One important use for nitric acid is the manufacture of various organic nitro compounds, especially explosives, such as trinitrotoluene (TNT), nitrocellulose, nitroglycerin, and RDX and PETN (the last two being components of Semtex). For example, nitroglycerin (the explosive component of dynamite) is made by adding nitric and sulphuric acids to glycerol under very carefully controlled conditions. Nitric acid is also used as an oxidising agent in the manufacture of nylon.
Nitrates
The biggest (80%) use of nitric acid, however, is in making ammonium nitrate, NH4NO3. This is an ingredient in many gunpowder recipes, and is an important explosive in its own right, but is mainly used as an agricultural fertiliser. It is also the parent of many other nitrates (compounds containing the NO3- ion), which are very rarely found in natural rocks since their high solubility in water means any deposits simply get washed away. Although such nitrate fertilisers have provided great benefits to mankind in increasing the food yield from otherwise poor soils, they have recently received a good deal of bad press. This is again due to their high solubility, which means the excess nitrates are readily washed into streams. There, they continue their job of promoting the growth of plantlife, particularly algae which reproduce unchecked to form smothering masses. The respiration of the algal masses use up the oxygen in the water, suffocating all other plant and animal life.
...and Nitrites
Nitrites, (compounds containing the NO2- ion), on the other hand, are made from nitrous acid (HNO2). Sodium nitrite (NaNO2) is used as a preservative in several processed meats, such as bacon, ham, sausage, corned beef, beef jerky, frankfurters, and some fish products. The nitrite ions (NO2-) in these meats inhibit the growth of a bacterium (Clostridium botulinum) which causes the fatal food poisoning known as botulism. It is also used because it fixes the bright red colour of fresh meat, which would otherwise quickly fade to an unpalatable brown.
 | Nitrites keep meat looking red.
|
However, there is controversy at the moment as to the toxicity of these nitrite additives, since it is known that when nitrite-cured meats are fried or boiled they can produce nitrosamines (compounds containing -NH-NO- groups) which are among the most powerful cancer-causing agents known. The counter argument is that removal of the nitrite preservatives would result in epidemics of food poisoning. The result of this controversy has been a reduction in the allowed concentration of nitrites in meat products, however it has still to be proven that nitrites cause cancer in animals.
 The oxides of nitrogen, nitric oxide, NO, and nitrogen dioxide, NO2, are both collectively known as NOx, and are familiar as one of the several components of automobile exhaust that create smog. In the hot combustion chambers of a car's engine, N2 and O2 react together to form NO. This process is normally unfavourable, but at high temperatures in the combustion chamber the reaction proceeds rapidly. The NO passes into the exhaust with the other gases, and then cools so rapidly that the NO can't revert back into nitrogen and oxygen again, because the reaction rate becomes too slow at these lower temperatures. Once released into the atmosphere, NO reacts to form NO2, which is familiar as the brown hazy colour of smog which is often seen clinging above modern cities. The problem gets worse, however, since NO2 can then be split apart by ultra-violet radiation from the sun, to produce oxygen atoms. These then react with oxygen molecules to make ozone. On calm days during summer, this ozone can build up close to the ground in cities, causing numerous respiratory problems among pedestrians.
The oxides of nitrogen, nitric oxide, NO, and nitrogen dioxide, NO2, are both collectively known as NOx, and are familiar as one of the several components of automobile exhaust that create smog. In the hot combustion chambers of a car's engine, N2 and O2 react together to form NO. This process is normally unfavourable, but at high temperatures in the combustion chamber the reaction proceeds rapidly. The NO passes into the exhaust with the other gases, and then cools so rapidly that the NO can't revert back into nitrogen and oxygen again, because the reaction rate becomes too slow at these lower temperatures. Once released into the atmosphere, NO reacts to form NO2, which is familiar as the brown hazy colour of smog which is often seen clinging above modern cities. The problem gets worse, however, since NO2 can then be split apart by ultra-violet radiation from the sun, to produce oxygen atoms. These then react with oxygen molecules to make ozone. On calm days during summer, this ozone can build up close to the ground in cities, causing numerous respiratory problems among pedestrians.


