 | Michael Faraday - the discoverer of benzene |
The hydrocarbon that we now call benzene was first isolated in 1825 by Michael Faraday from an oily film that deposited from the gas used for lighting. Faraday did some experiments, and discovered that the new compound had equal numbers of carbons and hydrogens, and so named it 'carbureted hydrogen'. Another chemist, Laurent, proposed that due to it being discovered in illuminating gas, it should instead be called pheno, from the Greek phainein, meaning to shine. This name never really gained acceptance, but persists to this day as phenyl - the name for the C6H5- group.
 | Michael Faraday - the discoverer of benzene |
Nine years after its discovery, another chemist, Mitscherlich, found he could produce the same substance by heating a chemical that had been isolated from gum benzoin - so he decided to call the compound benzin. Other chemists rejected this name because it implied the compound was similar to alkaloids, such as quinine. Another suggestion was the German name, benzol, from the German öl, meaning oil. But in France and England the name benzene was used instead, to avoid the -ol ending confusing it with an alcohol.
The fact that benzene had a formula (C6H6) that suggested a polyene structure (i.e. many double- or triple-bonds), but did not behave at all like the other polyenes was an enigma for 19th Century chemists. In fact, whereas all the other known polyenes (e.g. butadiene) were highly reactive, benzene was remarkably inert. It became increasingly clear that there was something fundamentally different about benzene, and the family of related molecules that were gradually being synthesised from it. Because of their characteristic smells, these relatively inert, benzene-like compounds became known as aromatic compounds.
In 1865, Kekulé suggested that the structure of benzene was a regular hexagon with a hydrogen at each corner. "My mental eye..could now distinguish larger structures of manifold conformations; long rows, sometimes more closely fitted together; all twisting and turning in snake-like motion. But look! What was that? One of the snakes had seized hold of its own tail...". Later on he modified this theory to treat benzene as a mixture of cyclohexatrienes in rapid equilibrium: "...the form whirled mockingly before my eyes...".
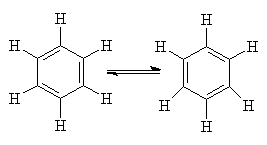
Cyclohexatriene
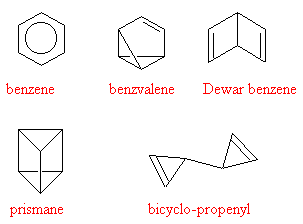
But such a structure should be highly reactive, and so didn't account for the unreactive nature of benzene. Other structures that were proposed at the time were Armstrong's centroid structure and Ladenburg's prismane.
 |  |
| Armstrong's 'centroid' | Ladenburg's prismane |
|---|
We now know that the best representation for the structure of benzene is indeed, hexagonal, with each C-C bond distance being identical, and intermediate between those for a single and double bond. The p-orbitals from each neighbouring C overlap to form a delocalised molecular orbital which extends around the ring, giving added stability and with it, decreased reactivity.
 delocalised p-orbitals
delocalised p-orbitals
Thus, benzene is often written as:  or
or 
The extra stability that is conferred by the resonance energy makes benzene by far the most stable compared to some of the valence isomers of C6H6 shown below. Their relative stabilities are in the order benzene > benzvalene > Dewar benzene > prismane > bicyclo-propenyl, and their energies from highest to lowest differ by almost 546 kJ/mol.
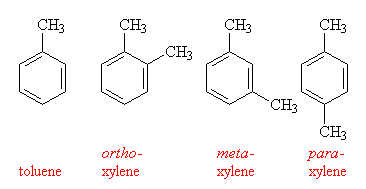
Toluene is simply benzene with an extra methyl group added to the ring, and is well-known as the precursor to TNT. It got its name because it was originally obtained from the gum of the South American tree Toluifera balsamum. This balsum, commonly called Tolu balsum, is a yellowy-brown with a pleasant aroma, and has been used in perfumes and cough syrup. On the other hand, the xylenes (from the Greek xulon for wood, since they were once obtained by distilling wood in the absence of air), have 2 methyl groups attached to the ring. There are 3 possible isomers of xylene, ortho-xylene, where the to methyls are next to each other, meta-xylene where they are separated by a hydrogen atom, and para-xylene where they are on opposite sides of the ring. A mixture of the 3 isomers is called xylol and is often used as a solvent.
Both toluene and xylene occur in gasoline (along with benzene). Their concentrations can be deliberately increased during the refining process to give a high performance BTX gasoline used for sports cars.