Ammonia (NH3) is a colourless pungent gas that is familiar to us as the smell of urine. In fact probably no other compound can be identified by its smell and correctly named by as many people as ammonia. It can be detected in the air at a level of only about 50-60 ppm, and at levels of 100-200 ppm it sharply irritates the eyes and lungs. At even higher concentrations it makes the lungs fill with fluid and can quickly cause death. Ammonia takes it name from the worshippers of the Egyptian god Amun - the Ammonians, because they used ammonium chloride (NH4Cl) in their rites. Ammonium chloride (also known as sal volatile) occurs naturally in cracks near volcanoes, and when it is warmed it decomposes into the pungent ammonia.
Industrially ammonia is made by the Haber-Bosch process which converts nitrogen gas into the air into ammonia. This process was discovered by the German chemists Fritz Haber (nobel prize 1918) and Karl Bosch, just in time for the beginning of WW1. This had important consequences for the length of the war, since without this process Germany would not have been able to make explosives (since it had no natural sources of nitrates from which explosives were made), and the war might have ended much sooner than it did.
| The Haber-Bosch Process - which takes place at 400-500°C and about 200 atm pressure, in the presence of an iron catalyst. |
In the mid-1980s, the annual production rate for ammonia was about 16 million tons. About 25% of this went directly for fertiliser, and the rest was used to make nitric acid (and from there into explosives), dyes, pharmaceuticals and cleaning agents. It has a relatively high heat of vaporisation, and so some ammonia is used as the heat-exchanger gas in large refrigeration units (rather than the ozone-destroying CFCs). With all of these important applications, it is no surprise that more molecules of ammonia are produced each year than any other industrial chemical.

| Most ammonia goes to make fertilisers which are used to improve the yield of crops in modern farms. |
Biologically, ammonia is a toxic waste-product of the breakdown of some amino acids, which it why it is converted to urea in the liver and then excreted in the urine. Once outside of the body, microbial action decomposes the urea back to ammonia, giving lavatories their familiar, unwelcome odour. Over-ripe Camembert or Brie also smell of ammonia, because it is formed as the nitrogen-containing proteins in the cheese decompose. But ammonia is also a useful fertiliser, since the nitrogen atom is in a much more reactive form than when in molecular N2, and so plants can absorb it readily and incorporate it into their own amino acids. Ammonia is also important as the precursor to a whole range of organic amines, such as amino acids and proteins.
Ammonia dissolves very readily into water because it can form strong hydrogen bonds with the water molecules. This is one of the reasons that we can smell it so easily, since it readily dissolves in the watery mucus in the nose. In solution, ammonia forms the ammonium ion (NH4+), one of the most important versions of which is found in ammonium nitrate (NH4NO3). This is another very important fertiliser, and is also used as an explosive.
 Hydrazine is a highly reactive liquid that can be made by reduction of ammonia with H2. Its main claim to fame is as a rocket fuel, since it burns in oxygen to produce hot gaseous products (steam and N2), releasing a lot of energy. The hot gases blast out of the rocket exhaust, so propelling it upwards. The same reaction can be used in a more controlled way in 'fuel cells', which convert the chemical energy in the fuel directly into electrical energy, this time with little or no waste heat being produced as a by-product.
Hydrazine is a highly reactive liquid that can be made by reduction of ammonia with H2. Its main claim to fame is as a rocket fuel, since it burns in oxygen to produce hot gaseous products (steam and N2), releasing a lot of energy. The hot gases blast out of the rocket exhaust, so propelling it upwards. The same reaction can be used in a more controlled way in 'fuel cells', which convert the chemical energy in the fuel directly into electrical energy, this time with little or no waste heat being produced as a by-product.
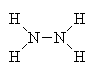 | Hydrazine - rocket fuel |