 | 
|
| The opium poppy, papaver somniferum | The seed pod, showing the exudate |
Opium is obtained from the opium poppy (papaver somniferum) by scraping the unripe seed capsule, and then collecting and drying the rubbery exudate. The name opium comes from the Greek opion, or poppy juice. It is important as a painkilling drug in its own right, but it is also the source of other analgesic drugs such as morphine and heroin.
 | 
|
| The opium poppy, papaver somniferum | The seed pod, showing the exudate |
Mankind had discovered the use of opium by the time of the earliest written records. In fact, the first recorded use of opium as a painkiller was around 6000 years ago by the Sumerians, and Babylonian and Egyptian writings contain many references to the value of opium preparations for the relief of pain. Later on, in Eurasia there emerged a legend that Buddha cut off his eyelids in order to prevent sleep overtaking him, and where they fell there grew a herb which bore a nodding violet flower which was to give sleep and tortured dreams to all mankind. Thomas Sydenham, the 17th-century pioneer of English medicine wrote: "Among the remedies which it has pleased Almighty God to give to man to relieve his sufferings, none is so universal and so efficacious as opium". Nowadays, although opium is no longer regarded as a universal analgesic, it is still very important as the source of morphine, the drug that is still the most commonly prescribed by physicians for the relief of severe pain.
Raw opium contains some 20 alkaloid substances, one of which is morphine, in a typical yield of 10%. Morphine was first isolated in 1805 by Friedrich Sertürner, an apothecary's assistant in Paderborn, Germany, however its basic structure was not correctly determined until 120 years later. In the 1800s morphine (known then as laudanum) was a popular panacea and was available from grocers and markets. Later on its addictive qualities led to its use and availability being severely restricted.
Morphine is isolated from opium in large quantities (over 1000 tons per year), although most commercial opium is converted into codeine by methylation. Morphine acts as an anesthetic without decreasing consciousness, and it is one of the most powerful analgesics known. However it also suppresses the respiratory system, and high doses can cause death by respiratory failure. Its analgesic properties are related to the ability of the molecule to fit into and block a specific receptor site on a nerve cell. This eliminates the action of the pain receptor, preventing the pain signals reaching the brain. This is similar to the way in which the body's natural painkillers (endorphins and enkaphalins) work. The shape of the morphine molecule is crucial to its ability to exactly fit into the active site on the receptor - the 'lock-and-key' mechanism. The benzene group of the morphine molecule fits snugly against a flat section of the receptor protein, whilst the bent neighbouring group of carbon atoms fits into a nearby groove. This allows the positively charged nitrogen atom to attach to a negatively-charged group on the receptor, so locking the two molecules together.
Morphine blocks deep aching pain, but has no effect on the fast pain that results from injury. One side effect of the analgesic action is that the patient often gets a feeling of detachment from the world, along with euphoria and sometimes pleasure. It is this which makes morphine, and the other drugs related to it (such as heroin), attractive to those who want to use drugs for 'recreational' reasons. However all of these drugs are highly addictive, and the body rapidly gets acclimatised to their use, such that increasingly large dosages are required for the same effect.
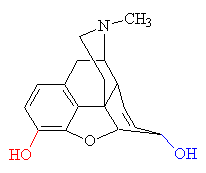 | Morphine - replacing the -OH group shown in red with -OCH3 produces codeine. Replacing the both the red and blue -OH groups with OCOCH3 produces heroin. |
 | Codeine - the methoxy addition is shown in red. |
 | Pethidine - the parts of the molecule resembling the morphine structure are shown in red. |
The isolation of morphine was the beginning of alkaloid chemistry, which has yielded many important medicinal substances. Although a satisfactory theory of analgesic structure or action still eludes us, experimenters have developed a number of synthetic analgesics related to morphine. The oldest is pethidine (also known as meperidine, Demerol and about 40 other names). It was synthesised in 1939 by the German chemist Otto Eisleb. It is less potent than morphine, but is still widely used for the relief of post-operative pain. By replacing one of the -OH groups with a methoxy group, morphine is converted into codeine, another powerful painkiller. When mixed with paracetamol it goes by the trade name Tylenol. When ingested, the -OCH3 group is converted back to -OH, regenerating morphine.
The most notorious derivative of morphine is heroin. It is synthesised by acetylation of the two hydroxyl groups of morphine with acetyl chloride, hence its other names, diacetylmorphine or diamorphine. Heroin was first isolated from morphine in 1874 and was originally used as a 'safe' (i.e. non addictive) cure from morphine addiction. This was later found to be incorrect and in the 1900s heroin abuse and addiction became common. Heroin was first restricted in the UK in 1868 due to 'unprofitable diversion of workers' and an international ban followed after the first world war. It is now classified as a Class A drug and possession or trading in it results in heavy penalties in most countries.

| Heroin The -OCOCH3 groups that replaced the -OH groups in morphine are shown in red. |
Replacing the hydrogen-bonding -OH groups with -OCOCH3 makes heroin much less soluble in water than morphine, but more soluble in non-polar solvents, like oils and fats. Therefore heroin has to be injected directly into the bloodstream, but once there it can pass rapidly through the blood-brain barrier which normally prevents the passage of water-soluble and large molecules. As a result it is much more potent than morphine, but its effect does not last as long. Again, once the heroin molecule is absorbed into the body, the acetyl groups are removed, reforming morphine.
Methadone hydrochloride is an opioid (a completely synthetic molecule), as opposed to an opiate, which is the name given to drugs which derive directly or indirectly from the opium poppy, such as morphine and heroin. It was originally synthesised by the German pharmaceutical company Axis during the second world war. It was first marketed as 'Dolophine' (to honour Adolph Hitler) and was used as an analgesic for the treatment of severe pain. It is still occasionally used for pain relief, although it is more widely used now as a substitute drug for people addicted to opiates (primarily heroin).

| Methadone - the parts of the molecule resembling the morphine structure are shown in red. |
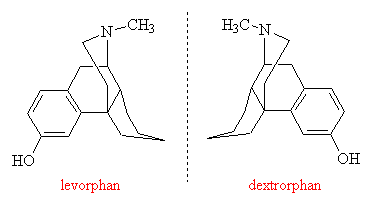
The fact that painkilling activity depends greatly upon the exact geometry of the molecule is strikingly illustrated by the drugs levorphan and dextrorphan. They are very similar in structure to morphine, except that they are both mirror images of one another. The left-handed molecule, levorphan, is several times more potent than morphine and is strongly addicting. But the right-handed molecule, dextrorphan, has no analgesic ability and is non-addicting. This is also true for morphine, which is naturally left-handed. There is no naturally occurring right-handed form of morphine, but the Japanese chemist Kakuji Goto has synthesised it and found that it possesses none of morphine's biological properties.