A dichotomy is a division into two mutually exclusive, opposed, or contradictory groups. Consider the reaction below. The bicyclic pentadiene on the left could in principle open on heating to give the monocyclic [12]-annulene (blue or red) via what is called an electrocyclic reaction as either a six (red) or eight (blue) electron process. These two possibilities represent our dichotomy; according to the Woodward-Hoffmann (WH) pericyclic selection rules, they represent contradictory groups. Depending on the (relative) stereochemistry at the ring junctions, if one reaction is allowed by the WH rules, the other must be forbidden, and of course vice-versa. It is a nice challenge to ask students to see if the dichotomy can be reconciled.
I start the process by pondering the relationship between the two forms of the [12]annulene shown on the right. Are the representations shown in red or blue just resonance isomers (analogous to the Kekule forms of benzene), or something else? If the former, then they truly represent the same species; they are just different ways of representing the contributions to the wavefunction, and the dichotomy stands. But if they are in fact different species, then we can start to eliminate the apparent contradiction by stating that the red and the blue arrows actually represent different reactions, leading to different (albeit isomeric) products. In this scenario, the red and blue forms of the [12]-annulene are NOT resonance isomers but distinct valence bond isomers, with a positive energy activation barrier to their interconversion. To find out, let us start with the transition states for both processes:
| C2 symmetry | Cs symmetry |
|---|---|
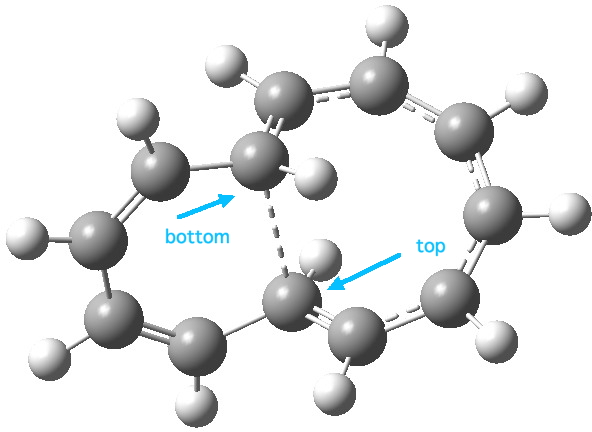 Transition state for blue arrows. Click for 3D. |
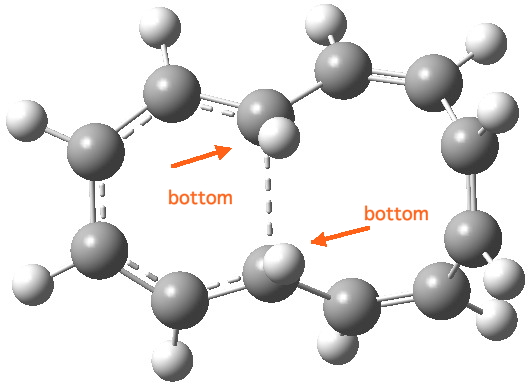 Transition state for red arrows. Click for 3D. |
- The blue arrows (representing 4n,n=2 electrons) result in a transition state with an axis of symmetry
- with the bond forming/cleaving from the bottom face of one terminus of the rhs-conjugated system to the top face of the other terminus, in other words an antarafacial bond,
- with conrotation of the groups at the termini, resulting in
- all the bonds in the 8-ring being approximately 1.4Å in length (other than the central bond), whilst those in the 6-ring alternate strongly. The 8-ring is (Möbius) aromatic and the 6-ring is (Möbius) anti-aromatic.
- Contrary-wise, the red arrows (representing 4n+2,n=1 electrons) result in a transition state with a plane of symmetry
- with the bond forming from the same bottom face of the lhs-conjugated termini, in other words a suprafacial bond,
- with disrotation of the groups at the termini, resulting in
- all the bonds in the 6-ring being approximately 1.4Å in length, whilst those in the 8-ring alternate strongly. The 6-ring is now (Hückel) aromatic and the 8-ring is (Hückel) anti-aromatic.
- The transition state with C2 symmetry is in fact 10.1 kcal/mol lower in free energy than the one with Cs symmetry.
| Product, C2 (axis) | Product, Cs (plane) |
|---|---|
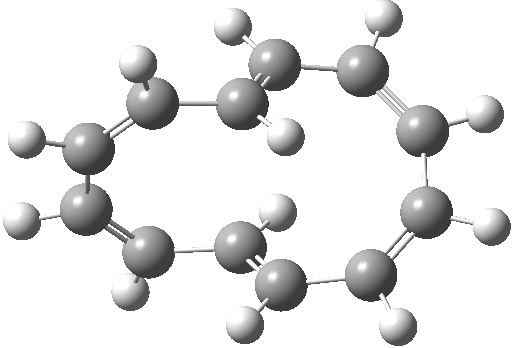 |
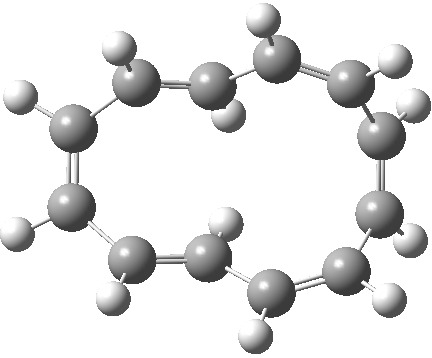 |
So at the end we see that there is no actual dichotomy. The reactions above (red or blue arrows) give different products, with different symmetries, and differently aromatic transition states. But in doing so, they encapsulate the selection rules for pericyclic reactions very nicely indeed. For more details of this, see this citation [1].
References
- H.S. Rzepa, "The Aromaticity of Pericyclic Reaction Transition States", Journal of Chemical Education, vol. 84, pp. 1535, 2007. http://dx.doi.org/10.1021/ed084p1535
Tags: Möbius, pericyclic, pericylic, positive energy activation barrier, Reaction Mechanism, Tutorial material