Many reaction mechanisms involve a combination of bond formation/cleavage between two non-hydrogen atoms and those involving reorganisation of proximate hydrogens. The Baeyer-Villiger discussed previously illustrated a complex dance between the two types. Here I take a look at another such mechanism, the methylation of a carboxylic acid by diazomethane.
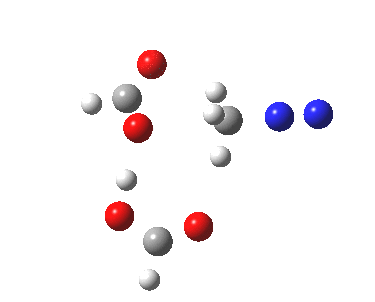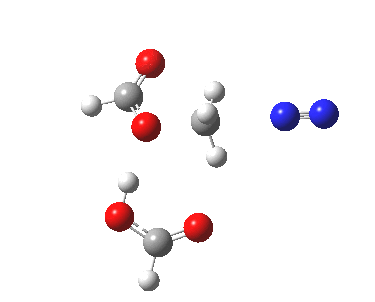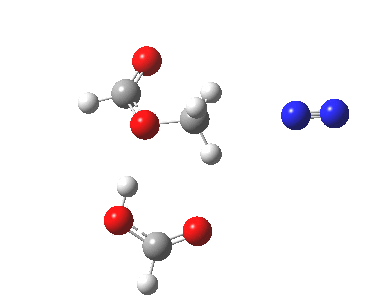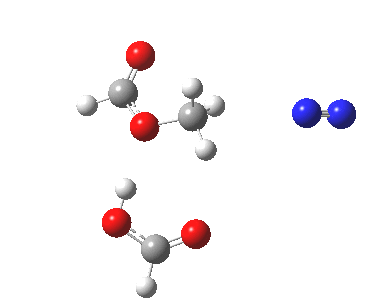
Text-books (or e-books) invariably show path (a). But the Baeyer-Villiger showed us that involvement of an additional acid as a proton transfer agent via a cyclic (7 or 11-membered) transition state was possible. So how about path (b, R=H), calculated using wB97XD/6-311G(d,p)/SCRF=dichloromethane?
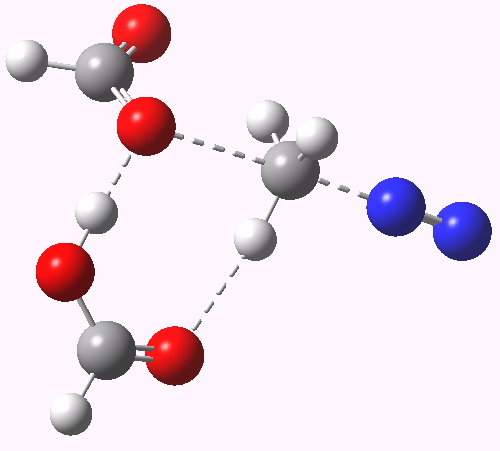
Path (b) in the diazomethane alkylation of a carboxylic acid. Click for 3D animation.
The IRC (intrinsic reaction coordinate) shows us the more detailed steps in the mechanistic dance. This tells us that the transition state shown above corresponds to the final stage of the reaction, path (c) in fact. The requisite reorganisation of the protons has already happened, and the reaction is happening from the zwitterionic intermediate shown in (c), with a barrier of only ~ 4 kcal/mol from that species.
 |
The transition state for the formation of the zwitterionic intermediate is itself shown below. One proton is clearly moving (to the carbon), but is the other? Again, an IRC is needed to tell us.
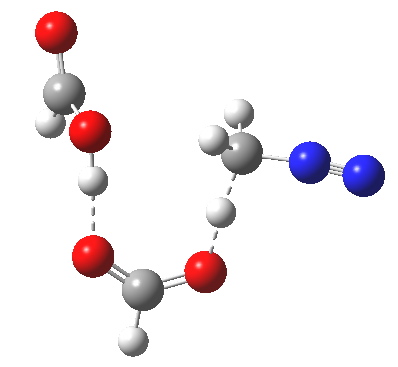 Transitions state for proton transfer, path (c). Click for 3D animation. |
|
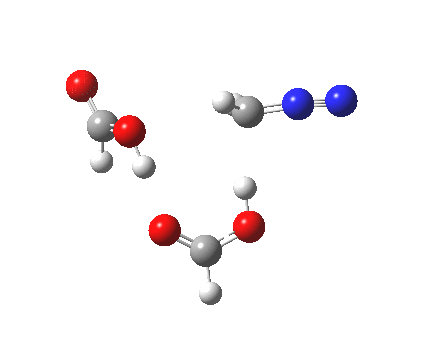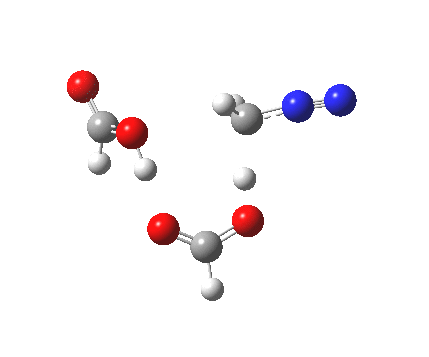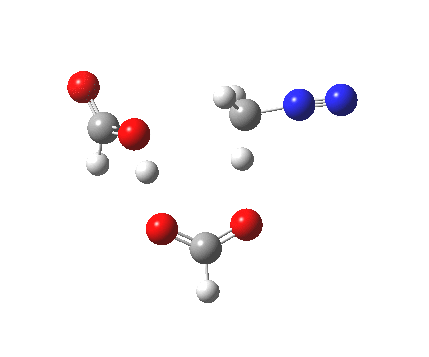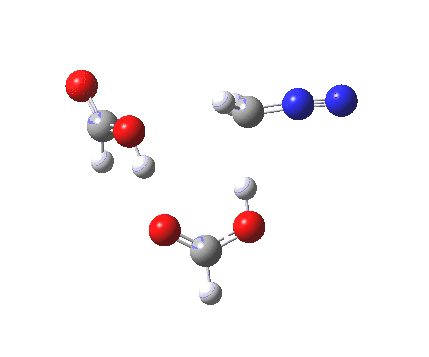 |
|
It seems that the two acid molecules do not co-operate with each other. The mechanism really does simply involve a protonation of the diazomethane by a molecule of acid to form a zwitterionic intermediate, following by attack by the anion of the acid on the diazonium cation to displace the nitrogen, path (a).
Tags: e-books, General, Tutorial material
[…] Mechanism of the diazomethane alkylation of a carboxylic acid. […]