March 21st, 2024
In the mid to late 1990s as the Web developed, it was becoming more obvious that one area it would revolutionise was of scholarly journal publishing. Since the days of the very first scientific journals in the 1650s, the medium had been firmly rooted in paper. Even printed colour only became common (and affordable) from the 1980s. An opportunity to move away from these restrictions was provided by the Web. Early adopters of this medium in chemistry were the CLIC pilot project[1] in 1995 and the Internet Journal of Chemistry (IJC), the latter offering “enhanced chemical publication which permits the publication of materials which cannot be published on paper and end-use customization which permits the readers to read articles prepared for their specific needs“.[2] Publication of the latter started in January 1998, offering “authors the opportunity to enhance their articles by fully incorporating multimedia, large data sets, Java applets, color images and interactive tools.” The journal remained online for seven years, after which it was closed and the articles became inaccessible. By then many major chemistry journals had started evolving along some of the same lines, and it could be argued this journal had served its purpose of alerting both publishers and authors to these new opportunities. Here I describe how an IJC article published in 2001 was brought back to life in more or less the enhanced manner intended.[3]
Read the rest of this entry »
References
-
D. James, B.J. Whitaker, C. Hildyard, H.S. Rzepa, O. Casher, J.M. Goodman, D. Riddick, and P. Murray‐Rust, "The case for content integrity in electronic chemistry journals: The CLIC project", New Review of Information Networking, vol. 1, pp. 61-69, 1995. http://dx.doi.org/10.1080/13614579509516846
-
S.M. Bachrach, "The 21st century chemistry journal", Química Nova, vol. 22, pp. 273-276, 1999. http://dx.doi.org/10.1590/S0100-40421999000200020
-
H. Rzepa, "Internet Archeology: an example of a revitalised molecular resource with a new activity now built in.", 2020. http://dx.doi.org/10.59350/9c769-34y25
Posted in Uncategorised | No Comments »
March 18th, 2024
I have written a few times about the so-called “anomeric effect“, which relates to stereoelectronic interactions in molecules such as sugars bearing a tetrahedral carbon atom with at least two oxygen substituents. The effect can be detected when the two C-O bond lengths in such molecules are inspected, most obviously when one of these bonds has a very different length from the other. The effect originates when one of the lone pair of electrons on one oxygen atom uniquely overlaps with the C-O antibonding σ* on another oxygen, thus shortening the length of the donating oxygen-carbon length and lengthening the length of accepting C-O bond. Here I take a look at tetra-substituted versions of this (C(OR)4), where in theory there are up to eight lone pairs, interacting with any of three C-O bonds, giving a total of 24 possible anomeric effects in one molecule.
Read the rest of this entry »
Tags: Interesting chemistry
Posted in Uncategorised | No Comments »
February 26th, 2024
The recent release of the DataCite Data Citation corpus, which has the stated aim of providing “a trusted central aggregate of all data citations to further our understanding of data usage and advance meaningful data metrics” made me want to investigate what the current state of citing data in the area of chemistry might be. Chemistry is known to be a “data rich” science (as most of the physical sciences are) and here on this very blog I try to cite whenever possible the source(s) of the data that I often use when discussing a topic. Such citations are not necessarily the same as citing a journal source via e.g. its DOI, although of course one is very likely to find data associated with most articles nowadays, albeit almost entirely via any associated supporting information document. However the latter is often presented in a relatively unstructured (PDF) form, which does not adhere to what are called the “FAIR” guidelines of being findable, accessible, interoperable and reusable. Directly citing data is a way of improving its FAIR-characteristics. So what insights does the Data citation corpus reveal? Read the rest of this entry »
Posted in Uncategorised | No Comments »
February 19th, 2024
Following on from my template exploration of the Wilkinson hydrogenation catalyst, I now repeat this for the Grubbs variant of the Alkene metathesis reaction. As with the Wilkinson, here I focus on the stereochemistry of the mechanism as first suggested by Chauvin[1], an aspect lacking in eg the Wikipedia entry. As before, the diagram below is hyperlinked to the appropriate data repository identifier so that you can go straight from the scheme to the data (Top level Data DOI: 10.14469/hpc/13796).
Read the rest of this entry »
References
-
P. Jean‐Louis Hérisson, and Y. Chauvin, "Catalyse de transformation des oléfines par les complexes du tungstène. II. Télomérisation des oléfines cycliques en présence d'oléfines acycliques", Die Makromolekulare Chemie, vol. 141, pp. 161-176, 1971. http://dx.doi.org/10.1002/macp.1971.021410112
Posted in Uncategorised | No Comments »
January 27th, 2024
In the late 1980s, as I recollected here[1] the equipment needed for real time molecular visualisation as it became known as was still expensive, requiring custom systems such as Evans and Sutherland PS390 workstations. One major breakthrough in making such techniques generally available on less specialised equipment was achieved by Roger Sayle[2], then working at Imperial College around 1990 and using a Silicon Graphics workstation. He greatly optimised up the rendering algorithms by creating a program called RasMol (after his initials), which meant such visualisations could very rapidly also be achieved even on a personal computer. Moving from vector display technology (the PS390) to Raster/bitmap graphics had allowed spacefilling representations of molecules containing 100s if not 1000s of atoms – and in turn enabled the new World-Wide Web to exploit the technique.[3]
Read the rest of this entry »
References
-
H. Rzepa, "Computers 1967-2011: a personal perspective. Part 2. 1985-1989.", 2011. http://dx.doi.org/10.59350/g4j62-4xk50
-
R. Sayle, "RASMOL: biomolecular graphics for all", Trends in Biochemical Sciences, vol. 20, pp. 374-376, 1995. http://dx.doi.org/10.1016/s0968-0004(00)89080-5
-
H.S. Rzepa, B.J. Whitaker, and M.J. Winter, "Chemical applications of the World-Wide-Web system", Journal of the Chemical Society, Chemical Communications, pp. 1907, 1994. http://dx.doi.org/10.1039/C39940001907
Posted in Uncategorised | 1 Comment »
January 25th, 2024
On 24th January 1984, the Macintosh computer was released, as all the media are informing us. Apparently, some are still working. I thought I would give my own personal recollections of that period.
Read the rest of this entry »
Posted in Uncategorised | No Comments »
January 21st, 2024
Geoffrey Wilkinson first reported his famous work on the hydrogenation catalyst that now bears his name in 1965[1] and I met him at Imperial College around 1969 and again when I returned there in 1977. He was still working on these catalysts then and I was privileged to collaborate with him on unravelling the NMR spectra of some of these compounds.[2],[3],[4]. During that period, computational modelling of the mechanisms of molecules containing transition elements was still in its infancy and I never extended my collaboration into this area at that time. Now, even if belatedly, I decided to explore this aspect and started to do this about two weeks ago. Here I thought that I would use this opportunity to show how I am going about it.
Read the rest of this entry »
References
-
J.F. Young, J.A. Osborn, F.H. Jardine, and G. Wilkinson, "Hydride intermediates in homogeneous hydrogenation reactions of olefins and acetylenes using rhodium catalysts", Chemical Communications (London), pp. 131, 1965. http://dx.doi.org/10.1039/C19650000131
-
K.W. Chiu, H.S. Rzepa, R.N. Sheppard, G. Wilkinson, and W. Wong, "Two-dimensional δ/J-resolved31P n.m.r. spectroscopy of [bis(diphenylphosphino)methane](trimethylphosphine)chlororhodium(I)", J. Chem. Soc., Chem. Commun., pp. 482-484, 1982. http://dx.doi.org/10.1039/C39820000482
-
C. Kwok W., C.G. Howard, H.S. Rzepa, R.N. Sheppard, G. Wilkinson, A.M. Galas, and M.B. Hursthouse, "Trimethyl and diethylphenylphosphine complexes of rhenium(I, III, IV, V) and their reactions. X-ray crystal structures of a bis(η5-cyclopentadienyl)-ethane-bridged dirhenium(I) complex obtained from phenylacetylene, tetrakis-(diethylphenylphosphine) (dinitrogen) hydridorhenium (I), tetrakis(trimethyl-phosphine) (η2-dimethylphosphinomethyl) rhenium(I) and tetrakis(trimethylphosphine) (iodo)methyl rhenium(III) iodide-tetramethylphosphonium iodide", Polyhedron, vol. 1, pp. 441-451, 1982. http://dx.doi.org/10.1016/S0277-5387(00)86558-4
-
K.W. Chiu, H.S. Rzepa, R.N. Sheppard, G. Wilkinson, and W. Wong, "Bis(diphenylphosphino)methane trimethylphosphine alkyl and η5-cyclopentadienyl compounds of rhodium(I); 31P{1H} two dimensional δ/J resolved and Overhauser effect nuclear magnetic resonance spectroscopy", Polyhedron, vol. 1, pp. 809-817, 1982. http://dx.doi.org/10.1016/0277-5387(82)80008-9
Posted in Uncategorised | 2 Comments »
January 12th, 2024
First, a very brief history of scholarly publishing, starting in 1665[1] when scientific journals started to be published by learned societies. This model continued until the 1950s, when commercial publishers such as Pergamon Press started with their USP (unique selling point) of rapid time to publication of ~3 months,[2] compared to typical times for many learned society publishers of 2 years or longer. Fast forward another 50 years or so, and the commercial publishers were now dominating the scene, but the business model was still based on institutional subscriptions, whereby the institution rather than authors paid the costs of publication. As the number of journals expanded, even well-off institutions had to make difficult decisions on which subscriptions to keep and which to cancel. By the late 1990s the delivery model was changing from print to online, but the overall issue was that many scientists around the world no longer had access to many journals.
Read the rest of this entry »
References
-
"Epistle dedicatory", Philosophical Transactions of the Royal Society of London, vol. 1, 1665. http://dx.doi.org/10.1098/rstl.1665.0001
-
D. Ginsburg, and W.J. Rosenfelder, "Alicyclic studies—X", Tetrahedron, vol. 1, pp. 3-8, 1957. http://dx.doi.org/10.1016/0040-4020(57)85003-0
Tags: Interesting chemistry
Posted in Uncategorised | 3 Comments »
January 8th, 2024
Zosurabalbin[1],[2], is receiving a great deal of attention as a new class of antibiotic which can target infections for which current treatment options are inadequate. It is a cyclic peptide and seeing this triggered memory of an earlier such species reported way back in 1995[3],[4]. This octa-peptide (YIJDIE, DOI: 10.5517/cc58gxs) was presumed to function in a novel manner, having linear water channels wide enough to form a molecular nanoscale pipe for a stream of water molecules to flow along. When inserted into the bacterial cell membrane via its lipophilic sidechains, it drained the bacterium of its cell water within seconds, thus killing it. A 3D model shows the effect very clearly.
Read the rest of this entry »
References
-
C. Zampaloni, P. Mattei, K. Bleicher, L. Winther, C. Thäte, C. Bucher, J. Adam, A. Alanine, K.E. Amrein, V. Baidin, C. Bieniossek, C. Bissantz, F. Boess, C. Cantrill, T. Clairfeuille, F. Dey, P. Di Giorgio, P. du Castel, D. Dylus, P. Dzygiel, A. Felici, F. García-Alcalde, A. Haldimann, M. Leipner, S. Leyn, S. Louvel, P. Misson, A. Osterman, K. Pahil, S. Rigo, A. Schäublin, S. Scharf, P. Schmitz, T. Stoll, A. Trauner, S. Zoffmann, D. Kahne, J.A.T. Young, M.A. Lobritz, and K.A. Bradley, "A novel antibiotic class targeting the lipopolysaccharide transporter", Nature, vol. 625, pp. 566-571, 2024. http://dx.doi.org/10.1038/s41586-023-06873-0
-
S. Hawser, N. Kothari, T. Valmont, S. Louvel, and C. Zampaloni, "2131. Activity of the Novel Antibiotic Zosurabalpin (RG6006) against Clinical Acinetobacter Isolates from China", Open Forum Infectious Diseases, vol. 10, 2023. http://dx.doi.org/10.1093/ofid/ofad500.1754
-
M.R. Ghadiri, K. Kobayashi, J.R. Granja, R.K. Chadha, and D.E. McRee, "The Structural and Thermodynamic Basis for the Formation of Self‐Assembled Peptide Nanotubes", Angewandte Chemie International Edition in English, vol. 34, pp. 93-95, 1995. http://dx.doi.org/10.1002/anie.199500931
-
S. Fernandez-Lopez, H. Kim, E.C. Choi, M. Delgado, J.R. Granja, A. Khasanov, K. Kraehenbuehl, G. Long, D.A. Weinberger, K.M. Wilcoxen, and M.R. Ghadiri, "Antibacterial agents based on the cyclic d,l-α-peptide architecture", Nature, vol. 412, pp. 452-455, 2001. http://dx.doi.org/10.1038/35086601
Posted in Uncategorised | No Comments »
December 29th, 2023
I will approach this example of a molecule-of-the-year candidate – in fact the eventual winner in the reader poll – from the point of view of data. Its a metallocene arranged in the form of a ring comprising 18 sub-units.[1] Big enough to deserve a 3D model rather than the static images you almost invariably get in journals (and C&EN). So how does one go to the journal and acquire the coordinates for such a model?
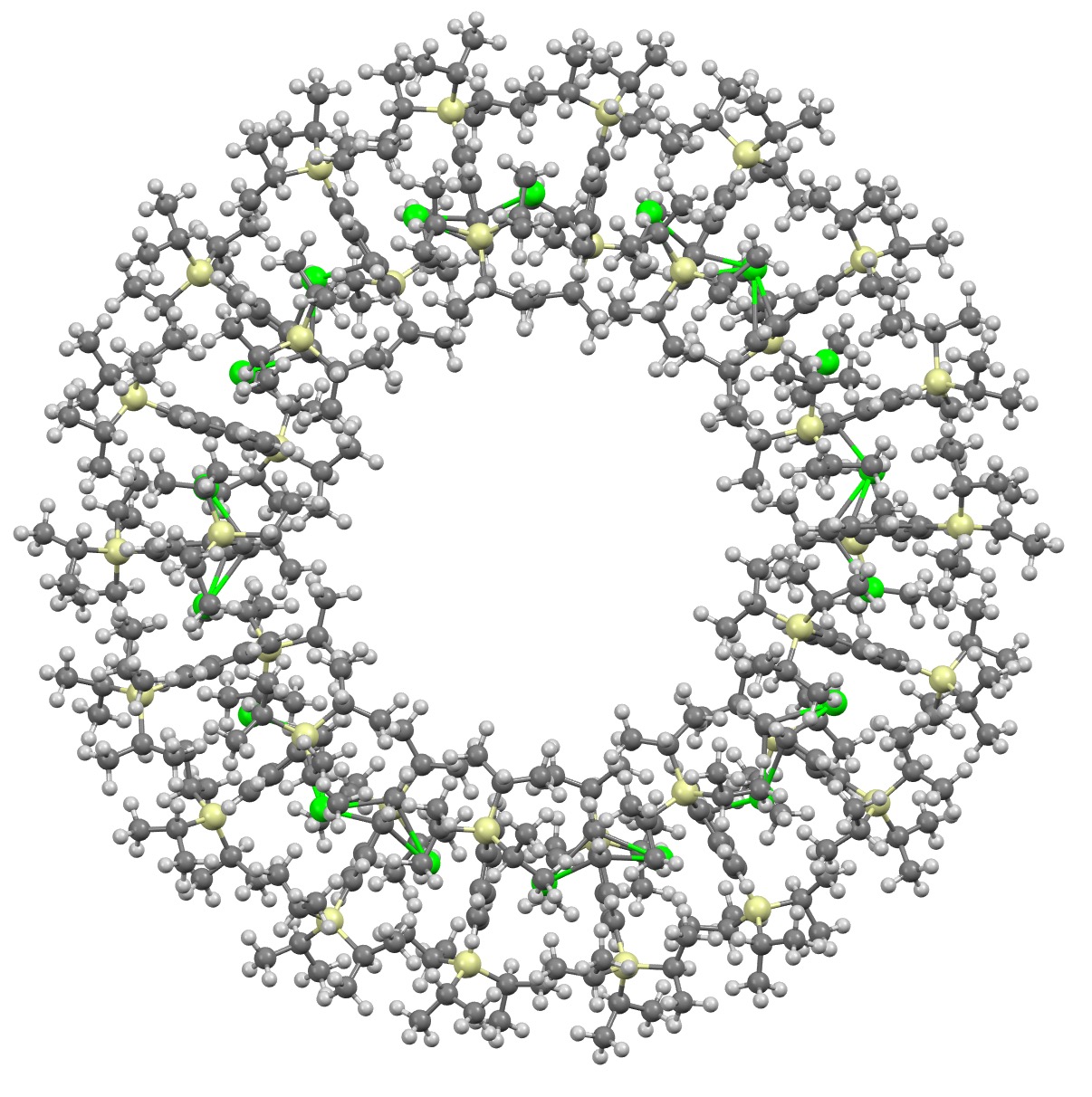
Read the rest of this entry »
References
-
L. Münzfeld, S. Gillhuber, A. Hauser, S. Lebedkin, P. Hädinger, N.D. Knöfel, C. Zovko, M.T. Gamer, F. Weigend, M.M. Kappes, and P.W. Roesky, "Synthesis and properties of cyclic sandwich compounds", Nature, vol. 620, pp. 92-96, 2023. http://dx.doi.org/10.1038/s41586-023-06192-4
Posted in Uncategorised | 2 Comments »
