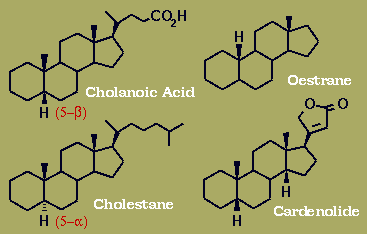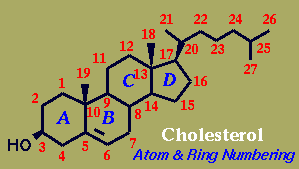
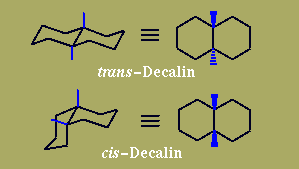
Naturally-occurring steroids have trans BC and - except for the cardiac glycosides - CD ring junctions, where cis/trans describes the relationship of the two acyclic bridgehead substituents. AB Junctions (represented here in the Decalin models) can be found either cis - as in Digitoxigenin or the bile acid Deoxycholic Acid - or more commonly as trans.