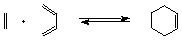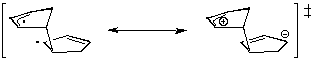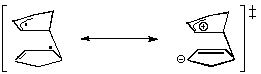Diels-Alder Reaction
- Introduction
Conjugated dienes undergo a cycloaddition reaction with multiple bonds to form unsaturated six-membered rings. In conventional terminology, this is a 1,4-addition of a diene and a dienophile. This reaction has a great synthetic importance and was discovered by two German chemists, Otto Diels and Kurt Alder, who received the 1950 Nobel Prize.
The simplest Diels-Alder Reaction is the reaction of
1,3-butadiene and ethylene to yield cyclohexene (Figure 1).
Figure 1 : Diels-Alder addition of ethylene and butadiene
- Reaction mechanism
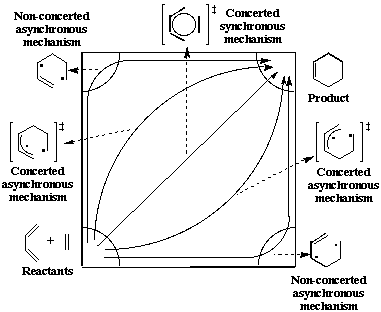
The Diels-Alder reaction is a thermal cycloaddition whose mechanism involves the sigma-overlap of the pi-orbitals of the two unsaturated systems. There is not a single mechanism for all Diels-Alder reactions[4]. At first approximation, we can divide them into two classes :
- Synchronous and symmetrical (concerted) mechanisms when the two new bonds are formed simultaneously. In the transition state, the two forming bonds have the same lengths. The combination of ethylene and butadiene is one example.
- Multistage (non-concerted) and asynchronous mechanisms. The transition state is a di-radical, one bond being formed, the other not.
Real mechanisms are a mixture of these two extremes, one bond being more properly formed and thus shorter than the other.
Figure 2 : Mechanisms of the Diels-Alder Reaction
To have an idea of the mechanism and to calculate the activation energy of a reaction, we have to find its transition state, using a gradient minimization.
The transition state of the Diels-Alder Addition of butadiene and ethylene shows that it looks like the reactants. It is called an early transition state.
- Reaction study
Here are the different computation results that we can get with the different computational methods :
| Unit : kCal/mol | Activation Energy
H‡ | Reaction Enthalpy
H‡
|
| MM2 | 23.76 | -56.45
|
| PM3 | 27.04 | -52.55
|
| AM1 | 23.76 | -56.45
|
Table 1 : Computation results for the Diels Alder additin of ethylene and butadiene
Experiments[7] give us the Arrhenius Activation Energy : 27.5 kCal/mol for a temperature between 760K and 921K. The Activation Enthalpy is then 27.5-(RT/4180)=25.83 kCal/mol (equation 10). Two remarks can be made on this result :
- The difference between the Activation Enthalpy and the Arrhenius Activation Energy is not so important. When this distinction is not meaningful, we will only refer to Activation Energy.
- There is a small difference between the PM3 and the AM1 value, but, as expected, the PM3 value is closer to the experimental result than the AM1 one. The difference with the experiments could be due to the temperature. The calculations of the activation energy and the reaction enthalpy are carried out at 298K.
Endo - Exo Selectivity
When both the diene and the dienophile are suitably substituted, a stereochemical feature arises because the reactants may approach each other in two distinct orientations. The substituent on the dienophile may be directed away from the diene (exo approach) or toward the diene (endo approach). This stereochemical difference is often found with cyclic compounds. One very simple exemple is the Diels-Alder addition of two cyclopentadiene(1) (Figure 3).
Figure 3 : Diels-Alder addition of two cyclopentadienes
- Mechanisms
In most Diels-Alder reactions, when the product distribution is under kinetic control, the endo adduct is preferentially, sometimes exclusively, formed. Several hypotheses have been made to explain this selectivity :
- Alder[5] proposed that endo addition was the consequence of a plane-to-plane orientation of diene and dienophile with "maximum accumulation of double bonds". Since this same orientation would promote stability in a molecular complex, it has been suggested that complex formation between the reactants may be responsible for preferential endo addition.
-
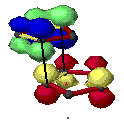
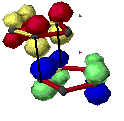
Woodward and Hoffmann[6] ascribe endo addition to interaction of occupied orbitals with unoccupied orbitals, the endo transition state conformation being favored by orbital symmetry relative to the exo conformation. With the model of frontier orbitals, we have interaction of HOMOs (Highest Occupied Molecular Orbitals) with LUMOs (Lowest Unoccupied Molecular Orbitals). Those interactions are represented in figure 4. The main interactions that will form the bonds are shown by plain bold lines, the secondary interactions, responsible for the endo-exo selectivity, are shown by small squiggly lines.
We do not know if the molecules approch so closely that the secondary interactions can be as important as shown in figure 1.
- Studies of Diels-Alder transition states can also give an explanation of this selectivity[7]. The transition state in an asynchronous mechanism can be described using several resonance structures (figure 5). One of this resonance structure is a zwitterion. In the endo transition state, the proximity of the two charges stabilizes this structure and thus favours the endo addition.
For the addition of the two cyclopentadienes, the forming bond lengths are 2.183 Å and 2.119 Å in the exo transition state and 2.184 Å and 2.127 Å in the endo transition state. This prove that the mechanism for this addition is asynchronous, and that the endo transition state is more assymetric, ie. the zwitterion resonance strucure is more favorized than in the exo attack.
Figure 4 : HOMO-LUMO interactions in the Diels-Alder addition of two cyclopentadienes
- Red lines represent single bonds
- Yellow lines represens double bonds
- Hydrogen have been omitted
|
| - Red and yellow orbitals represent the LUMOs
- Blue and green orbitals represent the HOMOs
|
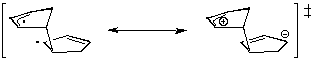 |
exo Transition State |
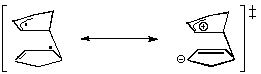 |
endo Transition State |
Figure 5 : Transition states in the Diels-Alder addition of two cyclopentadienes
- Results of calculations
| Unit : kCal/mol | Activation Energy
H‡ | Reaction Enthalpy
H°r
|
| endo | exo | endo | exo
|
| AM1 | 34.21 | 33.17 | -22.69 | -24.43
|
| PM3 | 37.40 | 36.61 | -19.34 | -21.20
|
Table 2 : Calculation results for the Diels-Alder reaction of two cyclopentadienes
We have here the opportunity to calibrate the results given by the semi-empirical program MOPAC that we used to calculate the activation energy, the reaction enthalpy and the geometry of the molecules and transition states. We see that calculation gives a small exo preference whereas only the endo-adduct is formed. We should be aware of this during the other experiments.
- Influence of the entropy
|
| H°r
(kCal/mol)
| S°r
(Cal/K/mol)
| G°r
(kCal/mol)
| H‡
(kCal/mol)
| S‡
(kCal/K/mol)
| G‡
(kCal/mol)
|
| PM3
| exo
| -21.20
| -48.39
| -6.80
| 36.61
| -44.18
| 49.85
|
| endo
| -19.34
| -48.31
| -4.92
| 37.40
| -44.43
| 50.57
|
Table 3 : Diels-Alder Addition of two cyclopentadiene
Detailed thermochemical parameters at 298K
The reaction entropy and activation entropy differences are very small and we can neglect their effect on the Gibbs Free-Energies G°r and G‡ (calculated using equation (2') and (9)). This is not surprising because the exo and the endo reactions are very similar. In both cases, we have the addition of two reagents that yield one product, and the bond transformations are identical. To compare Diels-Alder reactions, it is a good approximation to study only the reaction enthalpies (Hess' law (3) enables us to calculate them easily with the outputs of the calculations which are often heats of formation). The reaction enthalpy differences are almost the same as those of Gibbs free energy.
- Influence of the temperature
|
| H°r
(kCal/mol)
| S°r
(Cal/K/mol)
| G°r
(kCal/mol)
| H‡
(kCal/mol)
| S‡
(kCal/K/mol)
| G‡
(kCal/mol)
|
200 K
PM3
| exo
| -20.93
| -47.19
| -11.49
| 36.61
| -44.39
| 45.49
|
| endo
| -19.06
| -47.25
| -9.61
| 37.41
| -44.12
| 46.24
|
400 K
PM3
| exo
| -21.33
| -48.70
| -1.85
| 36.73
| -44.08
| 54.36
|
| endo
| -19.47
| -48.80
| 0.05
| 37.52
| -43.85
| 55.06
|
Table 4 : Diels-Alder Addition of two cyclopentadiene
Detailed thermochemical parameters at 200K and 400K
We have a confirmation that the yield of the Diels-Alder addition decreases with the temperature. With a temperature higher than 400K, we would favour the reverse reaction. This is due to the negative entropy that favours dissociation, and its importance increases with temperature. We observe that there is no change in the endo-exo selectivity, and that the entropy differences between endo and exo products and transition states are so small that we do not need to take them into account.
We conclude that we only need to deal with the heats of formation at 298K to predict endo-exo selectivity in the Diels-Alder additions. This does not let us have any ideas about the yield of such reactions, but only on the selectivity.
(1) Cyclopentadiene is a volatile hydrocarbon (b.p. 46°C), available commercially as a dimer that can be cracked thermally. The dimer boils at 170°C. When the free monomer has been prepared by slow distillation of the dimer, it must be used immediately as it re-dimerizes on standing to give the Diels-Alder endo-addition product.