 |
Localization of the transition states
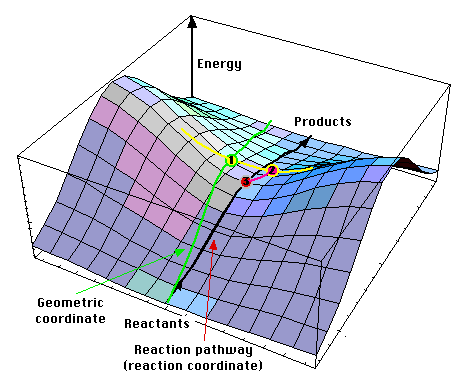
A transition states is always a first-order saddle point in the energy map. Energy rises in all directions but one. If energy would have lowered in two directions or more, then it is obvious that there would be an other path between reactants and products where every point is lower than this one (because the energy map is a continous surface).
 |
Localization of the transition states
| Previous | Table of Contents | Next |