Mauveine: The First Industrial Organic Fine-Chemical
Henry Rzepa, Department of Chemistry, Imperial College.
|
Whilst the origin of the modern industrial revolution is often traced to the Ironbridge
Gorge northwest of Birmingham in the UK, less credit is given to one of the birthplaces of
the modern organic chemical industry. William Henry Perkin (1838-1907) who at the age of 18
in the year 1856 had set out with idea of making quinine
(C20H24N2O2) by oxidising allytoluidine
(C10H13N), had accidentally produced instead the first ever synthetic
dye aniline purple, better known as mauveine. The actual formula of the two principal
components is C26H23N4+X -/
C27H25N4+X - X= sulphate, acetate,
chloride and the chemical name is e.g. 3-amino-2,9-dimethyl-5-phenyl-7-(p-tolylamino)phenazinium
acetate. It is worth noting that the molecular structures of few compounds were known with
certainty at this time. Even the tetravalency of carbon was not common knowledge at
this time, having been suggested by Kekule only in 1857, and by William Odling a little
earlier. Loschmidt in 1861 then published structures for
about 250 molecules known at the time. Many of these suggestions are now recognised as
corresponding precisely to the modern formulae. The list includes a number of
1,3,5-triazenes, which are represented in the modern cyclohexatriene manner of benzene, and
of course are structurally not dissimilar to the central mauveine ring system.
Loschmidt's structures predate Kekule's benzene structure by four years.
|
|
|
|
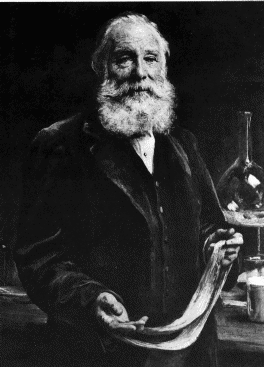 |
Perkin set up a factory (Perkin and Sons) on the banks of the Grand Union, then the
Grand Junction Canal, in 1857 to produce mauveine. This small dyeworks was located on a
6-acre site just south of the Black Horse public House, in Greenford, West London. This pub survives to this day, and remains a "local" for the
pharmaceutical giant, Glaxo SmithKline, which had its world headquarters nearby.
At the Royal Exhibition of 1862, Queen Victoria made an appearance in a silk gown dyed with
mauveine, thus replacing Tyrian purple, which had been restricted to royalty since Roman times due to its great expense. In the Imperial College chemistry archives, there is a sample of silk (approx 5 x 10 cm in size) dyed with a batch of the
original dye synthesised in the 1850s, and a "penny lilac" postage stamp originally
thought to have been dyed with the same compound. Curiously, the "correct" structure
for the compound was only finally put to rest as late as 1994, appropriately enough, in a journal named after Perkin himself |
|
| The new colour fell of of fashion in the late 1860s, but out of one of the world's
first chemistry "R&D" laboratories, Perkin discovered two new dyes, Britannia
Violet and Perkin's Green. The water in the nearby Grand Union Canal was said to
have turned a different colour every week- depending on what dyes were being made at the time.
In 1869, Perkin synthesised the vivid natural red dye called Alizarin, known chemically as
sodium 9,10-dihydro-3,4-dihydroxy-9,10-dioxoanthracene-2-sulfonate. The German company BASF beat
him to the patenting process by one day! Perkin and BASF came to an agreement over the
manufacturing processes. Perkin would sell Alizarin to Britain, some 400 tons a year, BASF to the
rest of the world, but the heyday of synthetic dye manufacturing in Greenford was now waning,
allegedly because British universities were not producing sufficient numbers of chemists for the
constant innovation now required in the dye industry. In 1874, Perkin sold his dyeworks to
Brooke, Simpson and Spiller. It continued operation under its new owners only until 1876, when it
was sold to the tar makers Burt, Boulton and Haywood, the dye operations of which joined the
British Alizarine Company. This in turn became part of ICI in 1931, and in more modern times
became known as Zeneca, a company which can claim to be the successor of Perkin.
|
|
Nowadays in Manchester, there remains a significant collection of interesting
chemicals and other Perkin artefacts, although they are not on permanent public view. Other
samples, including the original mauveine made by Perkin are located in the Science Museum,
London, just next to Imperial
College, and in the College archives themselves. Parts of the original Greenford buildings
survived until the centenary celebrations in 1957, and the last traces were demolished as
recently as 1976, the only remaining sign being the blue
plaque erected on the buildings now there. Nowadays, the site of the laboratory for
Perkin's dyeworks serves as a distribution point for
millions of loaves of bread and rolls for West London. The Black Horse pub itself has
recently been refurbished, and one can take a pleasure cruise along the Grand Union canal
starting from the pub. The east-bound cruise can take you to "Little Venice", in
Paddington, which is about 3kmn north of Imperial College.
After he retired in 1874, Perkin continued research in organic chemistry, discovering the
"Perkin Reaction", and perhaps most significantly, synthesising coumarin, which formed
the basis of the synthetic perfume industry. He published some 60 research papers during this
period, most of them in the Journal of the Chemical Society.
Photographs of the Dyeworks site, the Black Horse Pub, and surroundings:
Postscript 1
There is an interesting chemical postscript to the story of Mauveine. Essentially, its
synthesis was remarkable because it was based on a one-pot (dichromate) oxidation of a simple
(mixture) of aromatic (methyl)anilines, which are simple organic bases. Such bases are peculiarly
prone to producing intriguing products in apparently simple reactions. Scorpionine was made
recently in Charles Rees' group. Charles was the Hofmann Professor of Chemistry at Imperial
College, and hence a successor to August Hofmann (1818-1892) who was Perkin's original
supervisor and inspiration, and first professor of the Royal College of Chemistry (founded in
1845, now Imperial College) which Perkin attended. Appropriately enough Charles is wearing
a bow tie dyed with what is
apparently a sample of the original mauveine made by Perkin himself, and is of course holding the
journal named after Perkin. Scorpionine was made from the
reaction of S2Cl2 with di-isopropyl ethyl amine, and is itself another
so-called heteroaromatic compound, just like mauveine. Another simple base (DABCO, or
di-aza-[2.2.2]bicyclo-octane) was discovered in Henry Rzepa's group to react with Bromine to
give a very odd compound containing linear
chains of 7 bromine ions, the chain having an overall charge of 3-. Both Scorpionine
and the DABCO/Br2 adduct are highly coloured, just like mauveine, althogh neither
appears to have any utility as a dyestuff.
Postscript 2
To aid molecular discovery, the INChI identifiers for mauveine and alizarin
are included here as:
InChI=1/C27H24N4/c1-17-9-11-20(12-10-17)29-21-13-19(3)27-26(15-21)31(22-7-5-4-6-8-22)25-16-23(28)18(2)14-24(25)30-27/h4-16H,1-3H3,(H2,28,29)/p+1
1.12Beta/C14H8O7S/c15-11-6-3-1-2-4-7(6)12(16)10-8(11)5-9(22(19,20)21)13(17)14(10)18/h1-5H,17-18H,(H,19,20,21)/p-1
Postscript 3
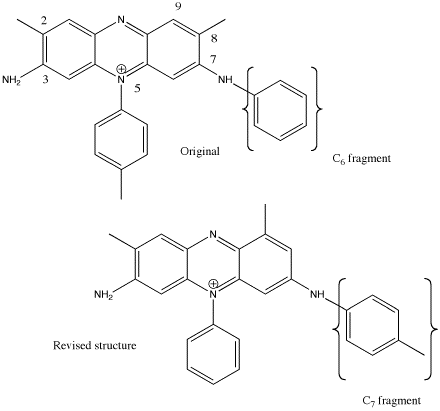 Although Perkin was the first to report so
called "crystalline" Mauveine acetate, a definitive X-ray determination of the
structure has never been reported. Meth-Cohn's1 analysis was based entirely on
spectroscopic analysis and analogies. It is of interest therefore to note that a recent paper
entitled "Molecular Tectonics. Selective Exchange of Cations in Porous Anionic
Hydrogen-Bonded Networks Built from Derivatives of Tetraphenylborate" appears to be the only
paper in the literature to report the structure of a molecule called Safranine (tetra-arylborate,
the point of interest having been the anion and not the cation, not the cation). Safranine has
two structural differences from mauveine. Firstly, in Safranine, the N-Tolyl group is replaced by
an NH2 and secondly the two methyl groups in Safranine occupy the 2,8 positions. The original
(pre-1994) structure of Mauveine also had methyl groups occupying these two positions. From this
it is apparent that Mauveine cannot be directly converted to Safranine, since that would result
in a methyl group in the wrong position. See DOI: 10.1021/ja042233m
Although Perkin was the first to report so
called "crystalline" Mauveine acetate, a definitive X-ray determination of the
structure has never been reported. Meth-Cohn's1 analysis was based entirely on
spectroscopic analysis and analogies. It is of interest therefore to note that a recent paper
entitled "Molecular Tectonics. Selective Exchange of Cations in Porous Anionic
Hydrogen-Bonded Networks Built from Derivatives of Tetraphenylborate" appears to be the only
paper in the literature to report the structure of a molecule called Safranine (tetra-arylborate,
the point of interest having been the anion and not the cation, not the cation). Safranine has
two structural differences from mauveine. Firstly, in Safranine, the N-Tolyl group is replaced by
an NH2 and secondly the two methyl groups in Safranine occupy the 2,8 positions. The original
(pre-1994) structure of Mauveine also had methyl groups occupying these two positions. From this
it is apparent that Mauveine cannot be directly converted to Safranine, since that would result
in a methyl group in the wrong position. See DOI: 10.1021/ja042233m
Further Information:
- The paper in which the correct structure was finally reported; O. Meth-Cohn and M. Smith,
J. Chem. Soc., Perkin Transactions 1, 1994, 5. DOI: 10.1039/P19940000005. This
paper revises the previously accepted structure in three ways.
- It places an aminotolyl substituent in the 7-position instead of a 7-aminophenyl
- The 5-substituent is now phenyl rather than the previous 5-tolyl
- A methyl group is now located at the 9-position rather than the 8-position (alternative
numbering; 1,8).
Some extra evidence for this revised structure can in fact be inferred from an article
Perkin himself wrote in 1879; W. H. Perkin, J. Chem. Soc. Trans., 1879,
717-732, DOI: 10.1039/CT8793500717. By combining "free mauveine" (we would
nowadays say this was the zwitterion) with HCl, and observing the change in mass, he
calculated a molecular weight of 406.7 (406.4 using modern weights); the formula
C27H24N4 (using modern atomic weights) would require 404.20.
This establishes with some certainty that mauveine vintage 1879 was the dimethyl form noted
above. Perkin then reports that treating this substance firstly with "peroxide of
lead" (this is probably PbO2) and then zinc dust, he obtains a species with
formula C20H18N4 he calls parasafranine. This amounts
to loss of C7H6, which corresponds to loss of the substituent at the
7-position; a substituent that can only be a p-Tolyl group (rather than a phenyl group,
C6H4). This firmly contradicts the structure extant until 1994, namely
that the p-Tolyl group was in the 5-position and that the 7-position carried a phenyl group.
It is rather odd that no-one spotted this during the period 1879 - 1994.
- A small-scale synthesis of mauveine is reported by R. L. Scaccia, D. Coughlin, D. W. Ball,
J. Chem. Ed, 1998, 75, 769.
- For a general account of the chemical history, see O. Meth-Cohn and A. S. Travis,
Chemistry in Britain, 1995, 31, 547.
- Anthony S. Travis "The rainbow makers: the origins of the synthetic dyestuffs industry
in Western Europe". Bethlehem: Lehigh UP, 1983.
- Henry S. Rzepa, Molecules, 1998, 3, 94-99.
- The Colour museum in Bradford and the Museum of Science
and Industry in Manchester
- Mauve: "How One Man Invented a Color That Changed the World", by Simon Garfield,
2000.
- On line biography
of Perkin, and some details of the chemistry of mauveine.
Acknowledgements
I am particularly grateful to Dr Peter Morris of the Science Museum, London, for very helpful
comments and corrections on the above history.
(c) H. S. Rzepa, 1995, 1998, 2001, 2004, 2006, 2017, 2019.






 Although Perkin was the first to report so
called "crystalline" Mauveine acetate, a definitive X-ray determination of the
structure has never been reported. Meth-Cohn's1 analysis was based entirely on
spectroscopic analysis and analogies. It is of interest therefore to note that a recent paper
entitled "Molecular Tectonics. Selective Exchange of Cations in Porous Anionic
Hydrogen-Bonded Networks Built from Derivatives of Tetraphenylborate" appears to be the only
paper in the literature to report the structure of a molecule called Safranine (tetra-arylborate,
the point of interest having been the anion and not the cation, not the cation). Safranine has
two structural differences from mauveine. Firstly, in Safranine, the N-Tolyl group is replaced by
an NH2 and secondly the two methyl groups in Safranine occupy the 2,8 positions. The original
(pre-1994) structure of Mauveine also had methyl groups occupying these two positions. From this
it is apparent that Mauveine cannot be directly converted to Safranine, since that would result
in a methyl group in the wrong position. See DOI:
Although Perkin was the first to report so
called "crystalline" Mauveine acetate, a definitive X-ray determination of the
structure has never been reported. Meth-Cohn's1 analysis was based entirely on
spectroscopic analysis and analogies. It is of interest therefore to note that a recent paper
entitled "Molecular Tectonics. Selective Exchange of Cations in Porous Anionic
Hydrogen-Bonded Networks Built from Derivatives of Tetraphenylborate" appears to be the only
paper in the literature to report the structure of a molecule called Safranine (tetra-arylborate,
the point of interest having been the anion and not the cation, not the cation). Safranine has
two structural differences from mauveine. Firstly, in Safranine, the N-Tolyl group is replaced by
an NH2 and secondly the two methyl groups in Safranine occupy the 2,8 positions. The original
(pre-1994) structure of Mauveine also had methyl groups occupying these two positions. From this
it is apparent that Mauveine cannot be directly converted to Safranine, since that would result
in a methyl group in the wrong position. See DOI: