News
The latest research outputs and goings-on
1 September 2023
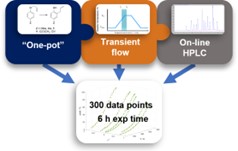
'One-pot' kinetics
Linden's multiparameter method for the collection of chemical reaction data for the Claisen rearrangement reaction is published in React. Chem. Eng.
[link to the article]

4 January 2024
Good start to 2024
Yifan won a prize at the 10th UK Catalysis Conference from Chemical Science, for his poster presentation: "High throughput catalysts screening of Ag-catalysed cyclisation of propargylic amides.". Congrats!

24 January 2024
Winning Double!
Huge congratulations to Matt and Linden for passing their PhD viva voce examinations. Well done, guys!
28 July 2023
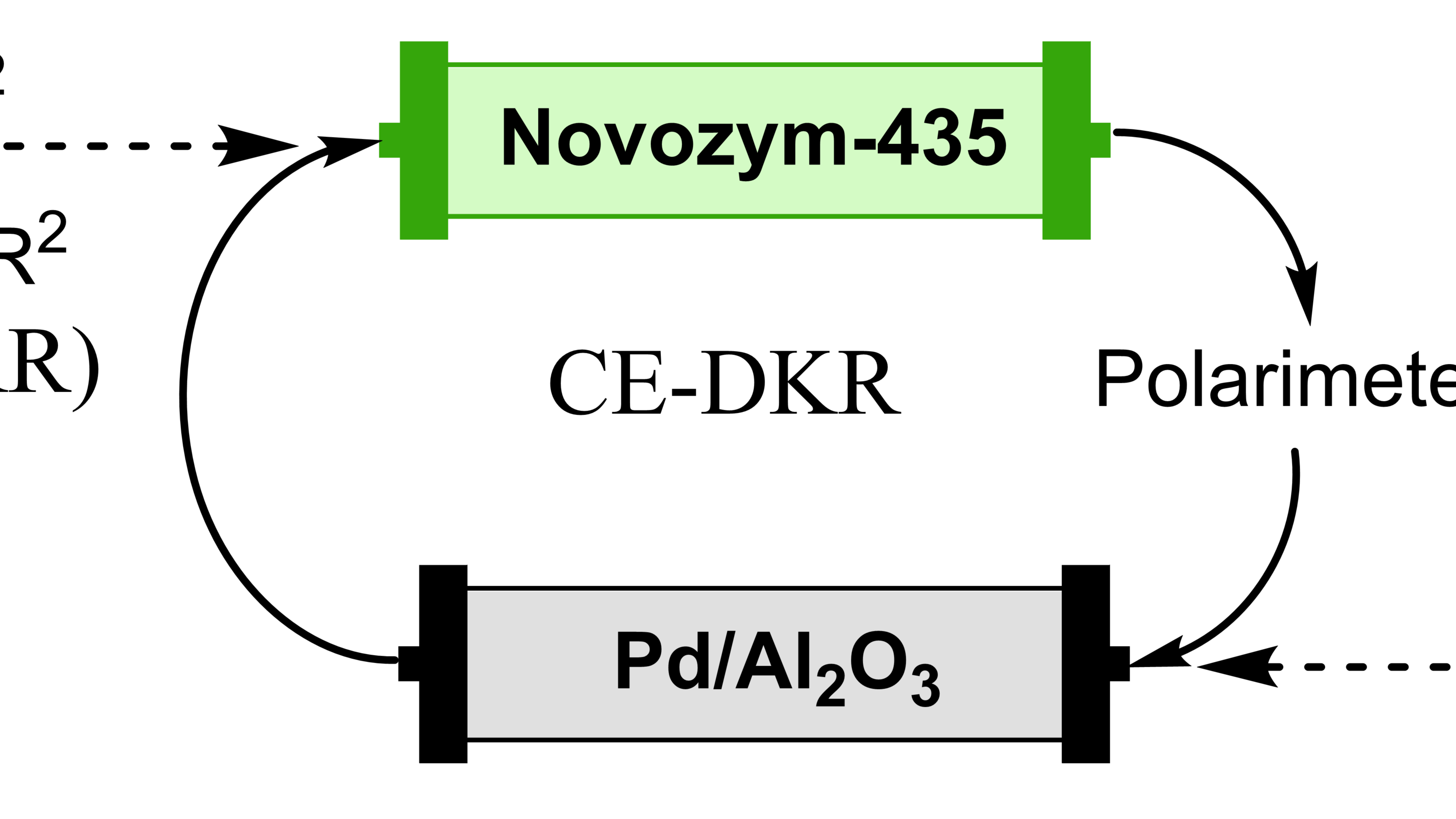
Matt's published!
Matt's work on the development of a Flash Thermal Racemization protocol for chemoenzymatic dynamic kinetic resolution is published in ACS Catal.
[link to the article]
