NMR Spectroscopy
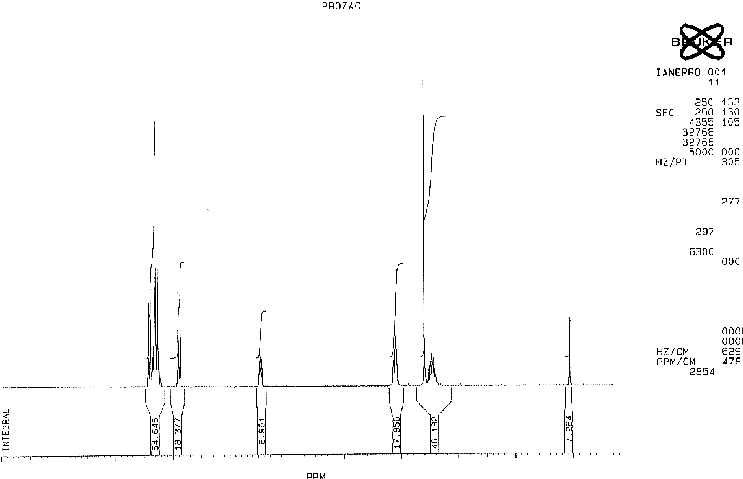
Analysis of peaks
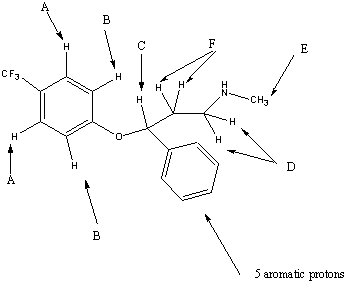
Chemical Shift/ppm | Assignment |
|---|---|
7.4 | A |
6.9 | B |
5.5 | C |
3.0 | D |
2.5 | E |
2.0 | F |


Chemical Shift/ppm | Assignment |
|---|---|
7.4 | A |
6.9 | B |
5.5 | C |
3.0 | D |
2.5 | E |
2.0 | F |
The A protons are chemically equivalent due to the symmetry of the system and hence appear at the same ppm value. Each A hydrogen will couple to its neighbouring B proton and the splitting pattern will be a doublet. The electron withdrawing effect of the fluorine atoms combined with the aromatic ring current is responsible for the high chemical shift of these protons.
The B protons are again in identical chemical environments and they each couple to the neighboring A proton. A doublet is therefore observed in the spectrum.
The C is close to an electronegative oxygen atom and an aromatic ring. These withdraw electron density both inductively and mesomerically. The C sisgnal is split into a triplet by 3J coupling to the adjacent F protons.
The D proons couple to the F protons but not to the adjacent NH proton resulting in a triplet.
The E signal corresponding to the methyl group is obviously a singlet as there are no adjacent hydrogens to which the E protons can couple.The NH proton is again discounted due to its transient nature.
The F protons show a multiplet. These protons are not chemically equivalent although they have been grouped together. These hydrogens are adjacent to an asymmetric centre and are thus diastereotopic. Replacement of each hydrogen by a different group would result in opposite diastereomers. As the F protons are not equivalent they couple to each other as well as to the D and C protons which are adjacent.A compliclated multiplet therefore results.
The hydrogen shown attached to the nitrogen atom does not appear in the spectrum as such protons readily interchange in solution.
The five aromatic protons appear as amultiplet between the shifts of the A and B protons.
 Top of page
Top of page