Peter Unwin
Introduction
The 1996 Nobel Prize for Chemistry has been won by Harold W. Kroto, Robert F. Curl and Richard E. Smalley for their discovery in 1985 of a new allotrope of carbon, in which the atoms are arranged in closed shells. The new form was found to have the structure of a truncated icosahedron, and was named Buckminsterfullerene, after the architect Buckminster Fuller who designed geodesic domes in the 1960's.
Formerly, six crystalline forms of the element carbon were known, namely two kinds of graphite, two kinds of diamond, chaoit and carbon(VI). The latter two were discovered in 1968 and 1972.
In 1990 physicists W. Krätschmer and D.R. Huffman for the first time produced isolable quantities of C60 by causing an arc between two graphite rods to burn in a helium atmosphere and extracting the carbon condensate so formed using an organic solvent.
The way was thus open for studying the chemical properties of C60 and other carbon clusters such as C70, C76, C78 and C84. New substances were produced from these compounds, with new and unexpected properties. An entirely new branch of chemistry developed, with consequences in such diverse areas as astrochemistry, superconductivity and materials chemistry/physics.

Professor Sir Harold W. Kroto
(University of Sussex)
Contents
1. Structure and physical properties of higher fullerenes pp.2-5
2. Production of fullerenes pp.6-8
3. Chemistry of fullerenes pp.8-10
1.) Structures and Physical Properties of Some Higher Fullerenes
Fullerenes consist of 20 hexagonal and 12 pentagonal rings as the basis of an icosohedral symmetry closed cage structure. Each carbon atom is bonded to three others and is sp2 hybridised. The C60 molecule has two bond lengths - the 6:6 ring bonds can be considered "double bonds" and are shorter than the 6:5 bonds.C60 is not "superaromatic" as it tends to avoid double bonds in the pentagonal rings, resulting in poor electron delocalisation. As a result, C60 behaves like an electron deficient alkene, and reacts readily with electron rich species. The geodesic and electronic bonding factors in the structure account for the stability of the molecule. In theory, an infinite number of fullerenes can exist, their structure based on pentagonal and hexagonal rings, constructed according to rules for making icosahedra.
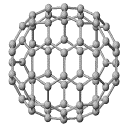
C60
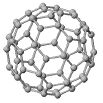 The structure of C60:
"Buckminsterfullerene"
The structure of C60:
"Buckminsterfullerene"
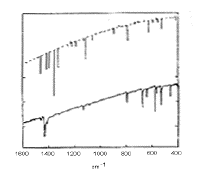
The infra red spectrum of C60(left); 13C NMR spectrum of C60
(right):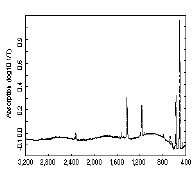
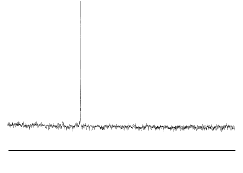
C60 only has four IR active vibrational bands, due to its icosohedral symmetry.
The UV/visual spectrum chromatographically purified C60 (left); A mass spectrum showing the higher fullerenes present in carbon soot (right):
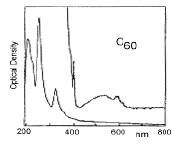
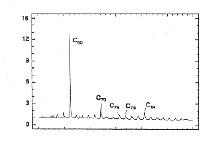
Mass spectrometry has been widely used to study the fullerenes. There is evidence for species as small as C20+, as well as stable peaks for the cluster ions C2n+ (where 2n>32).
Physical properties of C60:
Density: 1.65 g cm-3
Standard heat of formation: 9.08 k cal mol-1
Index of refraction: 2.2 (600nm)
Boiling point: Sublimes at 800K
Resistivity: 1014 ohms m-1
Vapour density: N/A
Crystal form Hexagonal cubic
Vapour pressure: 5 x 10-6 torr at room temperature
8 x 10-4 torr at 800K
Organoleptic properties:
Appearance
Buckyball soot: Very finely divided black powder
Fullerite: Brown/black powder
C60: Black solid
Odour: Odourless
The fullerenes are also found to be soluble in common solvents such as benzene, toluene or chloroform. If one shakes up some fullerene soot with toluene and filter the mixture, one obtains a red solution. As the solvent evaporates, crystals of pure carbon appear
An HPLC trace showing fullerenes found in carbon soot:
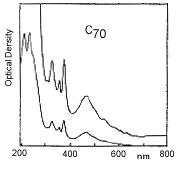
C70
 Structure of C70
Structure of C70
Infra red spectrum of C70 (left); UV/visual spectrum of C70 (right):
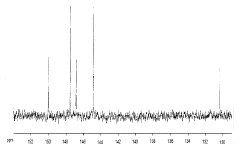
Carbon 13 NMR spectrum of C70:
C76
C84
"Buckytubes" & "Buckyonions"
Many fullerenes have now been discovered in carbon soot, uncovered by electron microscopy, including tubes of carbon many thousands of times long as they are wide, with the same icosahedral structure as the fullerenes. These have diameters as small as 2nm. Carbon "onions" have also been discovered, and consist of carbon cages one inside the other rather like Russian dolls. These carbon particles have millions of atoms, and many have been observed with dozens of concentric shells. Hypothetical structures have been postulated for carbon cages consisting of not pentagonal and hexagonal rings (like C60), but heptagonal (7 membered) rings.
A
helically coiled carbon nanotube
Atomic resolution picture of a single walled carbon nanotube taken using ascanning tunneling microscope.
2.) The Production of Fullerenes
The first method of production of fullerenes used laser vaporization of carbon in an inert atmosphere, but this produced microscopic amounts of fullerenes. In 1990, a new type of apparatus using an arc to vaporize graphite was developed in Germany by Kratschmer and Huffmann.
Adapted Kratcshmer-Huffmann apparatus as used at Widener University
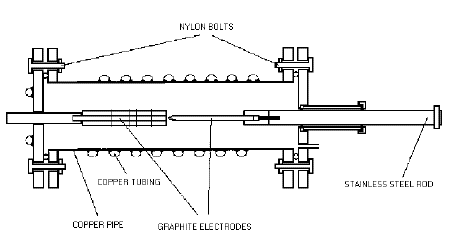
Method for fullerene synthesis
Pump down the system and introduce Helium gas into the chamber. Repeat (purge). Finally fill the bell-jar with about 100 Torr of Helium. Connect up the welding kit power supply Turn the on / off switch on the supply to the on position for 10 to 15 seconds. Afterwards there should be plenty of black soot like material produced inside the bell-jar. After a 5-10 min cool down period fill the bell-jar to atmospheric pressure. Take the bell-jar off and scrape the glass surfaces clean, collect all the material. 10 % of the soot should be made up of C60. The fullerenes in the soot are then extracted by solvation in a small amount of toluene.
After extraction, the solvent (toluene) is removed using a rotary evaporator, leaving behind a solid mixture of mostly C60 with small amounts of larger fullerenes. Pure C60 is obtained by
liquid chromatography. The mixture is dissolved in toluene and pumped through a column of
activated charcoal mixed with silica gel. The magenta C60 comes off first, followed by the red
C70. The different color solutions are collected separately and the toluene removed using the
rotary evaporator.
A Mechanism for Fullerene Formation
Although many mechanisms have been described, only the "pentagon road" appears to explain high yields of C60. This is described below, in an extract taken from an address by Professor Richard Smalley to the Robert A. Welch Foundation 39th Conference on Chemical Research: Nanophase Chemistry: Self-Assembly of Fullerene Tubes and
Balls.
"At the moment there is only one mechanism that appears to be consistent with yields of C60 as high as 30-40%. It is known as the Pentagon Road. In this view, the yield is high because clustering continues in a hot enough region to permit the growing clusters to anneal to the minimum energy path: one where the graphene sheet (a) is made up solely of pentagons and hexagons, (b) has as many pentagons as possible, while (c) avoiding structures where two pentagons are adjacent. If the pentagon rule structures really are the lowest energy forms for any open carbon network, then one can readily imagine that high-yield synthesis of C60 may be possible. In principal, all one needs to do is adjust the conditions of the carbon cluster growth such that each open cluster has ample time to anneal into its favored pentagon rule structure before it grows further. This path through the kinetics is the Pentagon Road, and constitutes a mechanism of graphene-sheet self-assembly that leads to C60 in very high yield.
The Pentagon Road must be the path that is followed in the high yield conditions. This requires that the cluster growth temperature be high enough to anneal the open structures as they grow so that they follow the pentagon road, but that the temperature not be so high as to permit the extensive rearrangement and mounting of high activation barriers necessary to convert the open pentagon road structure to the closed fullerene.
In the Krätschmer-Huffman (KH) experiment, carbon radicals are produced simply by slow evaporation of the surface of a resistively heated graphite rod. Here the carbon vapor density is far lower than that in pulsed laser vaporization, thus so too is the rate of clustering. Most critically, the rate of cooling of the condensing carbon vapor is much slower in the KH method. By adjusting the pressure of helium buffer gas around the evaporating graphite rods, one has at least coarse control over this rate of cooling and the clustering rate. At too low a pressure the carbon radicals migrate far away from the hot region around the resistively-heated rod and are too cold when they grow to the region of C60. But with just the right pressure of helium, the clustering in the critical size range occurs at just the right distance from the hot source that the temperature is optimal for the growing clusters to follow the Pentagon Road.
After the KH method was introduced, it was found at Rice that a simple ac or dc arc would produce C60 and the other fullerenes in good yield as well, and this is now the method used commercially. Even though the vaporization mechanism of a carbon arc differs markedly from that of a resistively heated carbon rod (because it involves a plasma) the optimum helium pressure for C60 formation is found to be very similar in each case. It is not so much the vaporization method that matters, but rather the conditions prevailing while the carbon vapor condenses. By adjusting the helium buffer gas pressure, one controls the rate of migration of the carbon vapor away from the hot graphite rod and thereby controls (at least crudely) the effective temperature and carbon radical density in the region where clusters in the size range near C60 are formed.
The Pentagon Road mechanism is just one of many by which fullerenes can form, but it may be the only one capable of accounting for the single most telling fact of fullerene formation: that the overall C60 yield can be as high as 40% of all the carbon vaporized. The Pentagon Road explains this as a consequence of annealing the open graphene sheets to their optimal open structure (given by the Pentagon Rule) at a rate faster than their growth, while avoiding rearrangement to a closed fullerene prior to the size of C60. It is a case of picking the reaction conditions to favor a particular product."
Commercial production of Fullerenes
The world's first computer controlled fullerene production plant is now operational at the MER corporation, who pioneered the first commercial production of fullerene and fullerene products.
Commercial uses of fullerenes
Although fullerenes are not yet used commercially, applications are being researched, such as catalytic methane activation to higher hydrocarbons funded by the US Dept of Energy. Other properties of fullerenes and their compounds such as superconductivity have yet to be exploited.
3.) Chemistry of the Fullerenes
The reactions and reaction types of the fullerenes described below are merely examples of some recent work, not an exhaustive study.
General
Redox chemistry
C60 and C70 have similar properties, with six reversible one electron reductions to C60(6-) and C70(6-) having been observed. Oxidation of C60 and C70 is, however irreversible. The first reduction for both fullerenes is ~1.0V for (Fc/Fc+), indicating they have electron accepting properties. C76 exhibits both electron donor/acceptor properties.
C60 is an "alkene"
C60 has a tendency of avoiding having double bonds within the pentagonal rings which makes electron delocalisation poor, and results in the fact that C60 is not "superaromatic". C60 behaves very much like an electron deficient alkene and readily reacts with electron rich species (see its organometallic reactions), but can also sometimes behave in a similar way to aromatic rings.
Classes of fullerene compunds
Compounds of fullerenes may be classed according to two different catagories: Exohedral (inside the cage) and Endohedral (outside the cage). Examples of the former include metals such as La enclosed in a C82 cage, and examples of the latter include transition metal complexes eg with Ir, as well as purely organic fragments bonded to the fullerene cage.
Organic Fullerene Chemistry
Fully brominated C60: C60Br(24)
As noted above, fullerenes behave as electron deficient alkenes, and will react with electron rich species, such as halogens. Formation of C60Br24 by reactio of solid C60 with neat liquid bromine takes 5-8 days, but recently (Sept. 1996) a new method has been developed at Widener University, which reduces the reaction time to about an hour.
Experimental : C60 (50mg) is placed in a closed conatiner with neat liquid bromine (5ml) and large gauge iron wire (ca. 1g), and stirred at room temp. for 1h. The excess liq. Br is then removed by vacuum evaporation, and the resulting solid treated with methanol to remove FeBr3 formed during the reaction. The remaining solid is then filtered and washed with methanol.
Mechanism: The mechanism is thought to be similar to that of electrophilic aromatic substiution, i.e. the in-situ formation of FeBr3, (a Lewis acid) catalyzes the reaction by polarizing the Br2 molecule for electrophilic attack.
 C60Br24
(ref. HADSIO)
C60Br24
(ref. HADSIO)
Electrophilic Aromatic substitution on C60: C60Ph5H
The structure of C60Ph5H, formed via Elelctrophilic Aromatic
Substitution. Reaction of C60Cl6 with benzene/FeCl3 yields C(60)Ph(5)Cl which is readily reduced to C(60)Ph(5)H, the main product of reacting C-60 with Br-2/FeCl3/benzene. Both compounds have been characterised by NMR spectroscopy.
(ref: Kroto, H.W. et al, J. Chem. Soc. Chem. Commun. 1994, 1463.)
C60Ph5H spacefill model (left) Crystals of C60Ph5H (right)
Organometallic Fullerene Chemistry
Endohedral Compounds
When C60 was first discovered, new experiments were rapidly devised to test the C60 (as a closed cage structure) hypothesis. As the C60 structure is hollow, with room for one or more other atoms, attempts were made to enclose a metal atom. A graphite sheet was soaked with a solution of a metal salt (lanthanum chloride, LaCl3) and subjected to vaporisation-condensation
experiments. Mass spectroscopic analysis of the clusters formed showed the presence of C60La+. These proved to be photoresistant, reinforcing the idea that metal atoms were "captured" inside the cage structure. The so called "shrink wrapping" experiment was then devised whereby ions of one size (or similar size) were captured in a magnetic trap and subjected to a laser pulse. The laser pulse causes the carbon cage to shrink by 2 carbon atoms at a time, until fragmentation ceased when pressure on the trapped metal atom is too big. The carbon cage now fits exactly around the metal atom. For example, (forC60Cs+) this size is at (C48Cs+) and for (C60K+) it is at (C44K+).
Below is an example of an endohedral metal fulleride, similar to that described above:
La@C82
This field of fullerene research is very active,as this recent abstract shows:
"Encapsulation of one or more metal atoms inside fullerene cages (endohedral metallofullerenes) is one of the most exciting topics in the fullerene science, since it could give rise to new species or materials with novel properties which are unexpected for hollow fullerenes. Recent important progress is marked by the successful isolation and purification of Sc@C-82, Y@C-82, La@C-82, Gd@C-82, La-2@C-80, and Sc-2@C-84 in large quantities. It is an interesting challenge to disclose how the properties of hollow fullerenes are modified upon endohedral metal-doping. Recently, we have clarified the electronic structures and properties of endohedral metallofullerenes based on the theoretical calculations and have undertaken the first exohedral functionalization of metallofullerenes with organosilicon compound which can act as a mechanistic probe to clarify the electronic and chemical character of fullerenes. These successful exohedral derivatizations of endohedral metallofullerenes should open the way to a variety of new chemical entities with novel properties, enriching the chemistry of metallofullerenes." - Akasaka, T et al Journal of Synthetic Chemistry Japan 1996, vol 54, no.7
Exohedral Compounds (C60 as a ligand)
Many exohedral compounds have been synthesized, such as the Pd and Ir complexes shown below. The fullerene ligands in these cases are acting in a similar manner to olefins.
 (eta$2!-C60-Fullerene)-bis(PPh3)-palladium (ref Hadsio)
(eta$2!-C60-Fullerene)-bis(PPh3)-palladium (ref Hadsio)
 eta.$2!-C60-Fullerene-bis
(benzoyloxybenzoyl (diphenyl) phospine) -chloro-carbonyl-iridium
(ref. kufhey)
eta.$2!-C60-Fullerene-bis
(benzoyloxybenzoyl (diphenyl) phospine) -chloro-carbonyl-iridium
(ref. kufhey)