Mechanism
.gif) Cisplatin enters cells by diffusion, where it is converted to its active form. This is due to the lower intracellular chloride concentration which promotes ligand exchange of chloride for water, and thus formation of the active aquated complex.
¤
The actual active species has not yet been determined with great certainty. There have been reports that the monoaquated species is the active form,
¤
which is dependent on pH - existing as the hydroxy form in basic medium, and proposals of a platinum dimer that is bridged by two hydroxyl groups [(NH3)2Pt(mu-OH)2Pt(NH3)2]2+ which forms prior to DNA interaction.
¤
However, the most common structure is considered to be the diaquated form, cis-diaquadiammineplatinum(II).
Cisplatin enters cells by diffusion, where it is converted to its active form. This is due to the lower intracellular chloride concentration which promotes ligand exchange of chloride for water, and thus formation of the active aquated complex.
¤
The actual active species has not yet been determined with great certainty. There have been reports that the monoaquated species is the active form,
¤
which is dependent on pH - existing as the hydroxy form in basic medium, and proposals of a platinum dimer that is bridged by two hydroxyl groups [(NH3)2Pt(mu-OH)2Pt(NH3)2]2+ which forms prior to DNA interaction.
¤
However, the most common structure is considered to be the diaquated form, cis-diaquadiammineplatinum(II).
The principle function of cisplatin is to bind to DNA. The consequence of this, is the activation of repair processes which eventually cause cell death. This explains why cisplatin is sometimes classed as an alkylating agent. Currently, the precise mechanisms that induce cellular apoptosis is not yet fully understood, however, some progress and insights have been made.
Although cisplatin is also able to interact with many types of proteins that are vital to DNA replication and cell division, its primary target remains to be DNA. Evidence supporting this is as follows:
¤
,
¤
- Observed filamentous growth in bacteria. This effect is almost certainly due to the selective inhibition of DNA synthesis since cell growth (i.e., RNA and protein synthesis) remains unaffected whereas cell division does not occur.
- Mutagenesis*. DDP has been shown to be mutagenic in both bacterial (prokaryotic) and human (eukaryotic) cells. The cis isomer is more mutagenic than the trans isomer, implying differences in their DNA binding. Further, repair-deficient mutants are more sensitive than those proficient in repair.
- Inactivation of viruses. The ability of platinum complexes to eliminate the infectious activity of extracellular papovirus SV40 indicates inactivation of the viral DNA.
- Inhibition of DNA synthesis. Compared to its effect in RNA and protein synthesis, cisplatin consistently and uniquely inhibits DNA synthesis both in vitro and in vivo.
- Selective binding to DNA. Assessment of platinum binding to macromolecules from cultured cells at known levels of cell killing and adjustment for molecular weight differences showed that more platinum was bound to DNA.
*This may sound contradictory to the previous page, and it may be. There remains an uncertainty as to whether cisplatin is either actually mutagenic (and carcinogenic for that matter) or not. The mutagenicity of the drug has not yet been confirmed officially. As it stands, many including myself, do believe cisplatin to be mutagenic, however, this requires us to clearly define this "property". If the definition includes physical alteration of DNA as well as chemical, then cisplatin can indeed be considered a mutagen.
The active aquated species is a bifunctional electrophilic agent
¤
and is therefore able to bind to any nucleophilic site present on DNA. Of the four nucleic acid residues, cisplatin has been shown to preferrentially associate with guanine.
¤
|
|
|
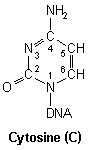
|
|
|
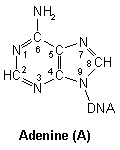
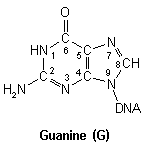
Purines
|
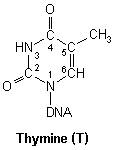
Pyrimidines
|
|
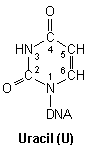
DNA nucleic acid residues
|
Closer inspection of each base reveals numerous nucleophilic atoms which cisplatin can bind to. Of the many potential sites available, cisplatin has been shown to selectively bind to only a few. Initially, it was reported that O6-Guanine (atom six of guanine), was the site of importance, being linked with both carcinogenesis and the mechanism of antitumour action.
¤
However, a year later, this proposal was amended
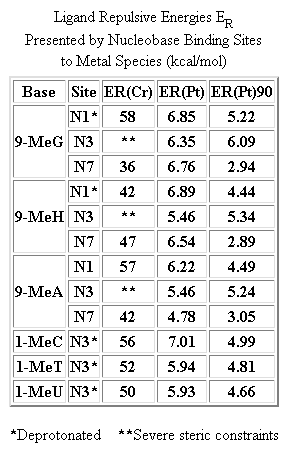
¤. Tests involving the action of cisplatin on a guanine-like structure (1,3,9-trimethylxanthine), showed it to bind at N7 and not O6. Further studies revealed cisplatin to preferrentially bind at N7 of the purines adenine and guanine, and at N3 of the pyrimidines cytosine and uracil. One research group,
‡
however, examined the steric effects between a closely related cisplatin compound triammineplatinum(II), and twelve possible binding sites present in a DNA strand. A "steric-probe" was used to calculate the lowest Van der Waals repulsive energy between the nucleobase and platinum complex, (table on right). Their findings showed N7-Guanine to be the most favoured site. This is now a generally accepted fact.
Being bifunctional in nature, cisplatin has the ability to bind to two nucleophilic sites. Considering the previous observations and discussions, it is not difficult to see that cisplatin will prefer to bind to two N7-Gunaine sites.
¤
,
¤
The kinetics of such a process has been shown not to be a simple two-step process.
¤
The reaction of cisplatin with GMP (Guanosine 5'-Monophosphate) was studied with a 500MHz NMR machine (1H & 31P). The data suggested a series of complex elementary steps, which are shown below.

Note that here, the active species is a monoaquated complex. The recorded values of the rate constants were k' (=ka + k1[GMP]) = 3.1x10-4s-1, k2 = 2.5x10-2dm3mol-1s-1, and k3 = 1.5x10-4s-1, for 1.0x10-3M cisplatin + 0.02M GMP at pH6.5. This shows that formation of the monoadduct is the fastest step.
Cisplatin does not bind just to N7-Guanine sites. It is able to bind to a large combination of bases, but to within certain restrictions. To form a biadduct, the two bases must not be more than one base apart. With the existence of DNA as a duplex, the binding of a base from each strand is possible, but only to those that are either directly opposite or diagonal to one another. The former type of biadduct is more commonly referred to as an intrastrand cross-link, while tha latter is referred to as an interstrand cross-link.

Studies in vitro, using gel mobility shifts, x-ray crystallography and other spectroscopic techniques, have shown cisplatin to react with DNA to form about 90% 1,2-intrastrand cross-links, of which about 65% are to two adjacent N7-Guanine sites [5'-d(GG)], and about 25% are to adjacent N7-Guanine and N7-Adenine sites [5'-d(AG)]. The remaining are other intrastrand cross-links, interstrand cross-links, monofunctional adducts and protein-DNA cross-links.
¤
,
¤
,
‡
,
‡
The high occurance of the intrastrand cross-link d(G*pG*) (platinum bound to two adjacent N7-Guanine sites), has been presumed to be due to minimal distortion and destabilisation, as they posses the shortest distance between two N7 atoms in DNA.
¤
.gif)
|
.gif)
|
|
|
|
Cisplatin bound to a DNA duplex (spacefill & sticks)
|
A widely accepted fact is that the formation of cross-links, is the cause of the drug's cytotoxicity.
¤
However, does the type of cross-link matter? Generally it does. Although it has not yet been possible to establish the relative contribution of each type of cross-link towards cisplatin cytotoxicty, it has been shown that intrastrand cross-links are the major contributors.
¤
,
‡
A classic example which shows this clearly is the comparative activity between cisplatin and transplatin.
¤
Cisplatin has long been shown to be significantly more cytotoxic than transplatin, forming predominantly intrastrand cross-links while transplatin predominantly interstrand.
¤
,
‡
Not surprisingly, not only does cisplatin form a lot of intrastrand cross-links, it also forms them much more quickly than interstrand cross-links.
‡
However, transplatin has been reported to be "kinetically more reactive than the cis isomer", and generally more kinetically labile, having a shorter half life than cisplatin.
¤
This has been used to explain its inactivity towards cancer, where the interstrand cross-links formed are "easier" to repair than intrastrand cross-links. (Dr. S. James, 1998)
Having identified d(G*pG*) (platinum bound to N7 of two guanine residues), intrastrand cross-links to be the most predominant and cytotoxic, the next step is to find out how and why. Many studies have concentrated on this area of research and all have made a common observation - the alteration of the physical structure of DNA when cisplatin is bound to it.
¤
,
¤
,
¤
,
¤
,
¤
,
‡
,
‡
One research group
¤
was able to produce an x-ray crystal structure of d(G*pG*) intrastrand cross-links. With the use of other spectroscopic techniques and a duplex DNA dodecamer, they were able to show the direct effects of cisplatin on DNA structure. The formation of d(G*pG*) adducts caused significant disruptions in base stacking to accomodate the coordination requirements of the square planar platinum(II) atom. The metal atom itself was displaced from the planes of the guanine bases by about 0.1nm and was considerably strained. Distortion of the double helix was severe, causing a degree of unwinding and significant bending of about 40° away from the site of attachment.
‡

The bound DNA strand was seen to affect the conformation of its complimentary strand in the form of a kink and a head to head anti-d(G*pG*) arrangement. The disruptions of the duplex structure were spread over several base pairs, however, the overall structure of the double helix remained intact. Most of the distortion was absorbed by conformational changes in the sugar-phosphate backbone near the platinum lesion.
[3D cDDP-DNA]
How does this physical alteration of DNA structrue cause cell death? Well, the current theory is programmed cell death. Cisplatin kills cells at any phase in the cell cycle, and is said to be phase-nonspecific, although there is now much evidence that it may be most effective in G1 of the cell cycle.
†
,
‡
It is generally believed that the alterations of the DNA structure prevents replication and thus activation of cellular repair mechanisms which are said to be the cause of eventual cell death. Initially, an attempt to salvage DNA is effected by excision- or mismatch- repair (MMR) mechanisms, whereby the faulty section of DNA is physically removed. This however, does not occur at an appreciable rate for fast dividing cells, therefore, cell cycle check-points detect the presence of defective DNA, and trigger a series of responses that lead to cellular apoptosis. An outline of these events, are as follows:
†
- Altered DNA prevents transcription
- P53 upregulated in response - Apoptosis
- P21 upregulated in response via both P53 dependent & independent pathways
‡
- Cell block at G1
- bax and other death agonists upregulated - Apoptosis
Clearly, the link between structure alteration and eventual cell death is prevention of DNA transcription. There are three mechanisms of action:
‡

-
Participation of HMG-box proteins.
‡
These High Mobility Group proteins have a great affinity for cisplatin-modified DNA, as much as 100 times than that for unmodified DNA.
‡
The reason is due to the resemblence of the bent DNA duplex as a binding site for these proteins.
¤
HMG-box proteins increase cisplatin cytotoxicity by binding onto DNA adducts and obstructing cellular excision repair.
- Irreversible platinum binding to transcription factors. Cisplatin is able to replace zinc(II) of a regulatory protein vital for the transcription of DNA with itself
‡. The so-called zinc-finger protein transcription factor, coordinates the bringing together of several cell components including part of the DNA strand and DNA-polymerase-alpha. The existence of the zinc cation is to allow amino acids of the protein, usually cysteine and histidine to coordinate towards it and thus pack together the nine small DNA binding domains into a dense structure.
¤
,
¤
Replacing the zinc ion with platinum, disrupts the conformation, and binds the "zinc"-finger permanently to DNA-polymerase-alpha, which is the transcription enzyme vital for cell replication.

The possible mechanism of cis-DDP binding to one of the nine zinc-finger domains
- Abortive correction repair. This can occur in many ways, however, it typically involves single or double stranded breaks caused by DNA polymerase and post-replicative repair mechanisms. DNA polymerase causes strand breaks by a physical process, whereby it literally collides with a platinum adduct. Post-replicative repair mechanisms produce nicks and breaks by mistaking the cisplatin adduct as a mismatched base pair. These strand breaks cause the upregulation of P53 and therefore, eventual apoptosis.
There is an indirect fourth mechanism of action which is the upregulation of P21 when using another drug etoposide, in conjunction with cisplatin. A study
¤
observed an upregulation of topoisomerases I & II, the roles of which are to form temporary single and double stranded breaks, respectively, to allow for un/winding of un/twisted DNA, and unknotting. An increase in concentration of these topoisomerases would therefore, indicate cisplatin to be causing knots and un/winding of DNA. The action of etoposide is to bind onto topoisomerase II and "lock" it in the "open" conformation, thus causing permanent DNA strand breaks.
†
This explains why the use of etoposide and cisplatin in combination chemotherapy produces excellent responses.
‡
Information of such mechanisms enables the design of new gene therapy strategies and developement of new and better platinum drugs that will be more potent, produce less side effects, and be effective against cisplatin-resistant tumours. This will be the final topic of discussion.

Copyright © 1998 Sai Man Liu
All Rights Reserved.
Last Modified on 24 June 1998
.gif) Cisplatin enters cells by diffusion, where it is converted to its active form. This is due to the lower intracellular chloride concentration which promotes ligand exchange of chloride for water, and thus formation of the active aquated complex.
¤
The actual active species has not yet been determined with great certainty. There have been reports that the monoaquated species is the active form,
¤
which is dependent on pH - existing as the hydroxy form in basic medium, and proposals of a platinum dimer that is bridged by two hydroxyl groups [(NH3)2Pt(mu-OH)2Pt(NH3)2]2+ which forms prior to DNA interaction.
¤
However, the most common structure is considered to be the diaquated form, cis-diaquadiammineplatinum(II).
Cisplatin enters cells by diffusion, where it is converted to its active form. This is due to the lower intracellular chloride concentration which promotes ligand exchange of chloride for water, and thus formation of the active aquated complex.
¤
The actual active species has not yet been determined with great certainty. There have been reports that the monoaquated species is the active form,
¤
which is dependent on pH - existing as the hydroxy form in basic medium, and proposals of a platinum dimer that is bridged by two hydroxyl groups [(NH3)2Pt(mu-OH)2Pt(NH3)2]2+ which forms prior to DNA interaction.
¤
However, the most common structure is considered to be the diaquated form, cis-diaquadiammineplatinum(II).


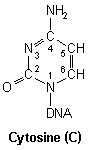




.gif)
.gif)


