Danishefsky's synthesis is convergent, with a key step being the coupling of an 'A' ring fragment with a 'CD' ring fragment to form the central 'B' ring (Figure 4).
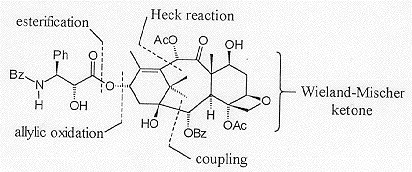
Synthesis of the enantiomerically pure 'CD' ring fragment starts with the Wieland-Mischer ketone 31, which is available as either enantiomer from a catalytic enantiospecific aldol condensation of a prochiral trione precursor. The conversion of 31 to 32 followed well established chemistry, and the early introduction of the oxetane proceeded smoothly to give 33 (Scheme 12).
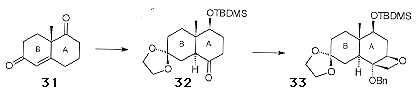
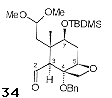
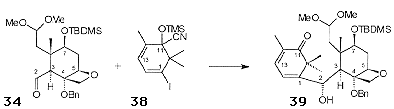
Pb(OAc)4-mediated oxidative cleavage of the 'B' ring of 33 allowed access to 34 (Figure 5), the required aldehyde coupling partner.
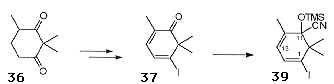
The 'A' ring precursor 38 was synthesised in 4 steps from the dione 36 (Scheme 13). Selective formation of the monohydrazone followed by the treatment with iodine and DBU gave rise to the iododienone 37, which was converted to the racemic cyanohydrin 38. Lithiation of 38 gave the nucleophilic fragment for coupling with aldehyde 34.
The initial coupling reactions carried out with the steroidal aldehyde 35 gave a single stereoisomer following hydrolysis of the cyanohydrin. Application of this protocol to the aldehyde 34 resulted in the isolation of 39 as a single stereoisomer (Scheme 14). Careful control of the reaction conditions was needed to avoid nucleophilic attack of the C2 hydroxyl on the oxetane ring.
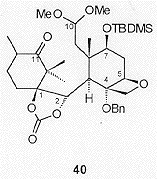
Directed epoxidation of the C14, C1 olefin in 39 followed by reductive cleavage gave a C1, C2 diol which, in common with Holton's total syntheses, was protected as a cyclic carbonate. Conjugate reduction of the resulting enone gave the cyclisation precursor 40 (Figure 6).
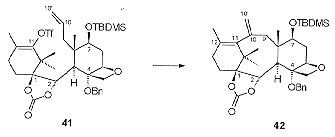
The 'B' ring was closed via an intramolecular Heck reaction. The 'A' ring ketone was activated as the enol triflate and the masked aldehyde at C10 was deprotected and methylenated to give 41 (Scheme 15). Treatment of 41 with stoichiometric tetrakis (triphenylphosphine) palladium resulted in ring closure to give 42 in 49% yield. The problem with the cyclisation reaction was found to be the olefin insertion /elimination steps, rather than the initial oxidative insertion into the C-OTf bond.
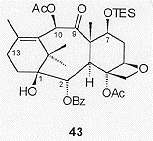
The oxidative cleavage of the C10, C10' methylene had been initially anticipated to be facile; however, it was soon found that the endocyclic C11, C12 alkene was preferentially oxidised with a range of oxidising agents, except dimethyldioxirane. Ultimately the endocyclic alkene was temporarily protected as an epoxide and the exocyclic double bond cleaved. Further protecting group manipulations, resulted in the cleavage of the carbonate with PhLi, and introduction of the C9 oxygenation led to the acetoxyketone 43 (Figure 12), which was also an intermediate in Nicolaou's approach.
Allylic oxidation to introduce the C13 oxygenation followed by diastereoselective sodium borohydride-mediated reduction of the C13 ketone gave 7-OTES-baccatin III which was then converted to Taxol®.
|
Glossary Bz = Benzoyl ; Ac = Acetyl ; Bn = Benzyl ; TIPS = Triisopropylsilyl ; TBS or TBDMS = tert-butyldimethylsilane ; TES = triethylsilyl ; BOM = benzyloxymethyl ; TMS = trimethylsilyl ; Tf or triflate = Trifluoromethanesulfomate
|
REFERENCE
Danishefsky. S.J : Am.Chem.Soc. (1996) 118, pp 2843- 2859