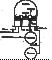
The following experiment describes the way to make buckminsterfullerene. The apparatus showed below is the original machine that was used to make the first quantities of C60 at Sussex University in 1989 - 90.( Document source is obtained from "University of Sussex, Fullerene Group Homepage")
Total experimental time - about 1 hour

Remove the glass bell-jar and if necessary fit some new carbon rods as described during the demonstration. Also arrange the KBr plates to within 6-7 cm from the rods.
Pump down the system (see the demonstrator) and introduce Helium gas into the chamber. Repeat (purge). Finally fill the bell-jar with about 100 Torr of Helium.
Connect up the welding kit power supply (NOTE: the welding kit power supply is a high current, low voltage supply and therefore there is little risk of electrical shock, however care should be taken as with any other 240 v mains operated device). Turn the on / off switch on the supply to the on position for 10 to 15 seconds. Afterwards there should be plenty of black soot like material produced inside the bell-jar.
After a 5-10 min cool down period fill the bell-jar to atmospheric pressure. Take the bell-jar off and scrape the glass surfaces clean, collect all the material. Believe it or not, 10 % of the soot should be made up of C60.
Take the soot covered KBr plate and measure the IR spectrum as directed by the demonstrator. You should be able to observe the tell-tale signs of C60, i.e. four weak absorptions on top of the larger absorption (see below).
Most molecules absorb in the Infra-red (IR) and the number of absorptions is dependant on the number of atoms in the molecule and how symmetrical the structure of the molecule is. In general an N atom molecule will have about 3N absorptions in the IR. As the symmetry increases the number of IR absorptions falls. For a molecule having 60 atoms one would expect 3 x 60 = roughly 180 IR absorptions. However because of the unique symmetry of Buckminsterfullerene, C60 has just 4 IR absorptions. This incredibly simple spectral fingerprint for C60 made IR spectroscopy a particularly effective method by which to probe for C60 in the arc made materials. We are going to use this technique to check that the carbon arc materials have C60 present in them.
Place a lot of the collected soots into a small flask. Add 20-30 ml of toluene and stopper the flask. Shake gently. Is there any colour change ? Next filter the solution. What colour is it ?
The fullerenes was just extracted from the soot. The coloured solution is due to a mixture of C60, C70 and larger fullerene cage molecules.
The next step is to purify the mixture of the fullerenes into separate fractions. Chromatography,which is the basis of the next experiments is used. To do the chromatography properly quite a lot of soot extracted solution is needed.
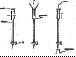
The chromatographic separation and purification of the fullerenes was first achieved at Sussex University soon after the carbon arc technique was discovered. Separation of pure allotropes by chromatography is believed to be unique to fullerenes.
Total experimental time - about 1 hour
Stage 1
Stage 2
Stage 3
Stage 4
Stage 5
The production of fullerenes can also be done in many other convenient ways. The example are the Solar Fullerene Production and the Pyrolytic Production of Fullerenes. The production of related fullerene is also done in the recent year. Example of this is the Fullerene Nanoprobes Production