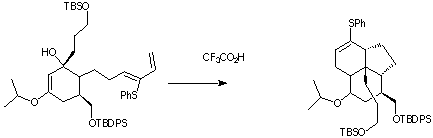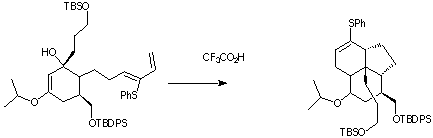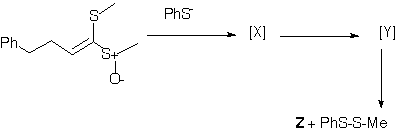Second Year Organic Problems. Set 5.
Qu. 1 . A key step in the synthesis of the natural product Lycopodine is shown below. Suggest a mechanism for this reaction, indicate clearly whether each step in the mechanism is a pericyclic or non-pericyclic process, and classify
each pericyclic process according to its type.
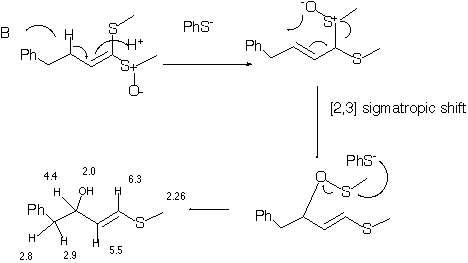
Qu 2 . In the sequence shown below, treatment of the reactant with sodium thiophenoxide (a base and also a thiophile) produces two transient isomers X and Y prior to the final product Z. The product Z has the following spectral
characteristics. nmax 3610 cm -1, 1H nmr d 2.0 (1H, broad s), 2.26 (3H, s), 2.8 (1H, m, J 13.6, 7.8Hz), 2.9 (1H, J 13.6, 5.8),4.40 (1H, J 7.8, 7.0, 5.8
Hz), 5.5 (1H, J 15.6, 7.0), 6.33 (1H, J 15.6), 7.4-7.6 (5H, m). Suggest a structure for Z, and a mechanism for its formation. Indicate clearly whether each step in the mechanism is a pericyclic or non-pericyclic process, and classify each
pericyclic process according to its type. Pay particular attention to the stereochemistry of Z
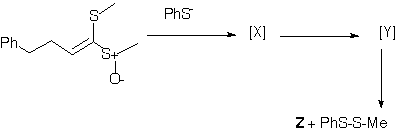
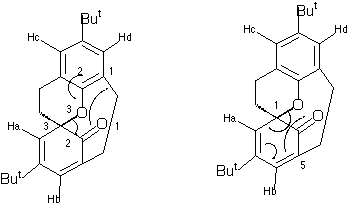
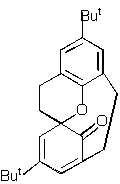
Qu.3. The 1H NMR spectrum of the compound shown below has some very peculiar properties. At -50C, four peaks are observed at d 5.73 and 6.25 (assigned to the olefinic protons) and at 6.95 and
7.04 (assigned to the aromatic protons). As the temperature is increased to about 20C, the two peaks due to the olefinic protons coalesce to a single peak, as do the two aromatic peaks. Further heating to 120C results in each now single
olefinic and aromatic peak further coalescing to a single peak, as do the two singlets due to the Bu t groups. Suggest pericyclic mechanisms to explain the behaviour observed at both 20C and 120C.
Second Year Organic Problems. Set 5. Answers.
Qu 1.
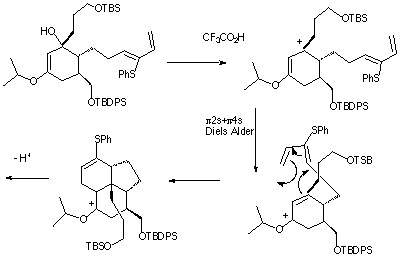
Qu 2 . Thiophenoxide acting as a base deprotonates to form an allylic anion stabilised by the adjacent sulfur groups. Reprotonation allows a [2,3]
sigmatropic rearrangement to occur
Qu 3 . The coalescence of NMR peaks on heating normally indicates a pericyclic process that renders protons equivalent. A [1,5] sigmatropic migration of the oxygen atom (with implied retention of configuration at this atom) will
exchange the environments of the protons labelled Ha and Hb, causing their NMR signals to coalesce. In fact, the process also
makes Hc and Hd equivalent.