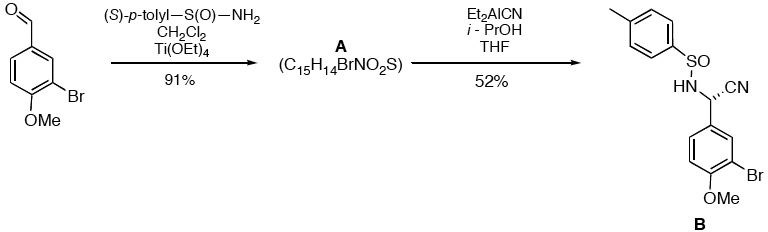
Synoptic Problems II. 2005
The question for this set and its answer
are available as Acrobat files. Molecular models corresponding
to the various systems are below. An article detailing the
anticipated mechanism for this reaction (the asymmetric
Strecker synthesis) is available here and reviewed here.
Models for B
In the models below, the aromatic ring substituents are not
included. Structure A is coordinated on the sulfoxide oxygen by
Me(OiPr)AlCN, which is formed by the reaction
between Me2AlCN and iPrOH. This
process attenuates the reactivity of this cyano transfer
reagent, the inference being that it is made more
selective.
The (monomeric) structure of the reagent is shown below,
together with that of the isocyano isomer. Note particularly
the unusual C-H...π double hydrogen bond that forms in each
case (toggle here to see atoms: ,), helping to stabilize the structure. There is
structural evidence that the "CN" group can coordinate to Al
via both an N and a C in a trimeric arrangement (check
this for yourself via a search of the Cambridge crystal
structure database), and that either Al-NC or Al-CN coordinated
monomers can result from disporportionation of this trimer.
| Relative energies (kcal/mol) based on
B3LYP/6-31G(d) Calculations |
| A, coordinated by Me(OMe)AlCN, 0.0
kcal/mol |
A, coordinated by Me(OMe)AlNC, +0.8
kcal/mol |
|
|
|
tris((μ2-Cyano)-bis(bis(trimethylsilyl)methyl)-aluminium:
YOYKIG |
|
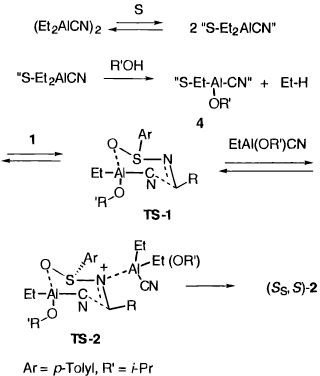 The two calculated
transition states for transfer of the CN group to the imine are
shown below. The (S,S)-stereoisomer is favoured over the (R,S)
diastereoisomer, largely the result of the latter having an
unfavourable steric interaction between the imine C-H hydrogen
and the proximate face of the phenyl group. The structure of
these transition states differs from that previously proposed in one important
regard. The original mechanism involved a six-membered ring
adopting a chair conformation, in which only the C of the CN
group transferred between Al and C (TS-1 in the scheme on the
left). When such a structure is subjected to calculation, it is
found to rearrange such that both the C and the N of the CN
group now form part of a larger 7-membered ring (). This ring size allows a more favourable angle of
attack by the nucleophilic C of the CN at the C=N double bond.
This implies that the coordinated reagent A first rearranges to
the isonitrile isomer, which is only slightly higher in energy
(via trimer formation and disproprtionation), and that this
isonitrile is actually the active species which attacks the
imine group.
The two calculated
transition states for transfer of the CN group to the imine are
shown below. The (S,S)-stereoisomer is favoured over the (R,S)
diastereoisomer, largely the result of the latter having an
unfavourable steric interaction between the imine C-H hydrogen
and the proximate face of the phenyl group. The structure of
these transition states differs from that previously proposed in one important
regard. The original mechanism involved a six-membered ring
adopting a chair conformation, in which only the C of the CN
group transferred between Al and C (TS-1 in the scheme on the
left). When such a structure is subjected to calculation, it is
found to rearrange such that both the C and the N of the CN
group now form part of a larger 7-membered ring (). This ring size allows a more favourable angle of
attack by the nucleophilic C of the CN at the C=N double bond.
This implies that the coordinated reagent A first rearranges to
the isonitrile isomer, which is only slightly higher in energy
(via trimer formation and disproprtionation), and that this
isonitrile is actually the active species which attacks the
imine group.
TS (S,R) 1.7 kcal/mol
2.3 [ΔG 1.3] (OMe) |
TS (R,R) 0.0 (barrier 18.1)
0.0 (OMe), barrier 21.3 |
|
|
The product is formed again as an μ2-cyano bridged
system, with the cyano bound to the imine carbon at the carbon,
and to the Al at the nitrogen. This system, when quenched with
water, results in protonation of the imino nitrogen, and
displacement of the μ2-cyano from the Al by another water
molecule, eventually resulting in eg Al(OH)3.
This type of alternative mechanism has previously been hinted at. Thus the guanidine catalysed Strecker reaction
is thought to proceed via addition of HNC rather than HCN to methanimine: 10.1021/jo034891f.
(C) H. S. Rzepa, 19/11/05.
 The two calculated
transition states for transfer of the CN group to the imine are
shown below. The (S,S)-stereoisomer is favoured over the (R,S)
diastereoisomer, largely the result of the latter having an
unfavourable steric interaction between the imine C-H hydrogen
and the proximate face of the phenyl group. The structure of
these transition states differs from that previously proposed in one important
regard. The original mechanism involved a six-membered ring
adopting a chair conformation, in which only the C of the CN
group transferred between Al and C (TS-1 in the scheme on the
left). When such a structure is subjected to calculation, it is
found to rearrange such that both the C and the N of the CN
group now form part of a larger 7-membered ring (). This ring size allows a more favourable angle of
attack by the nucleophilic C of the CN at the C=N double bond.
This implies that the coordinated reagent A first rearranges to
the isonitrile isomer, which is only slightly higher in energy
(via trimer formation and disproprtionation), and that this
isonitrile is actually the active species which attacks the
imine group.
The two calculated
transition states for transfer of the CN group to the imine are
shown below. The (S,S)-stereoisomer is favoured over the (R,S)
diastereoisomer, largely the result of the latter having an
unfavourable steric interaction between the imine C-H hydrogen
and the proximate face of the phenyl group. The structure of
these transition states differs from that previously proposed in one important
regard. The original mechanism involved a six-membered ring
adopting a chair conformation, in which only the C of the CN
group transferred between Al and C (TS-1 in the scheme on the
left). When such a structure is subjected to calculation, it is
found to rearrange such that both the C and the N of the CN
group now form part of a larger 7-membered ring (). This ring size allows a more favourable angle of
attack by the nucleophilic C of the CN at the C=N double bond.
This implies that the coordinated reagent A first rearranges to
the isonitrile isomer, which is only slightly higher in energy
(via trimer formation and disproprtionation), and that this
isonitrile is actually the active species which attacks the
imine group.