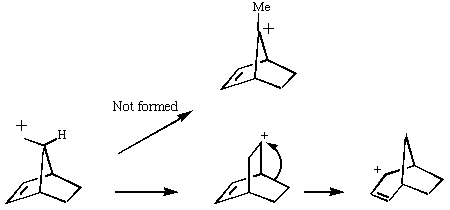
The only surprising result is that the MM method predicts that the tertiary carbonium ion is actually LESS stable than the preceeding primary ion, which is surprising to say the least. Clearly, electronic factors must be playing an important role as well. Suffice to say that even without ANY consideration of electronic factors, the MM2 method does not do too badly in its prediction of relative stabilities of this set of carbonium ions. This re-inforces the conclusion that bond angles in carbonium ions are just as important as substitution! Take a look for example at the angle contribution to the MM energies, which varies most. Also included are the semii-empirial (PM3) energies, which take into account lots of other factors, including effects such as hyper-conjugation, and p conjugation. In this particular system, the differences in these effects are only secondary in controlling the stability.
