| -25.4 kcal/mol | -24.7 kcal/mol |
|---|---|
2(b) Acyloxyboranes form by eliminating hydrogen betwen an OH part of the carboxyl group and BH3. This proceeds via an intermediate and a transition state 1
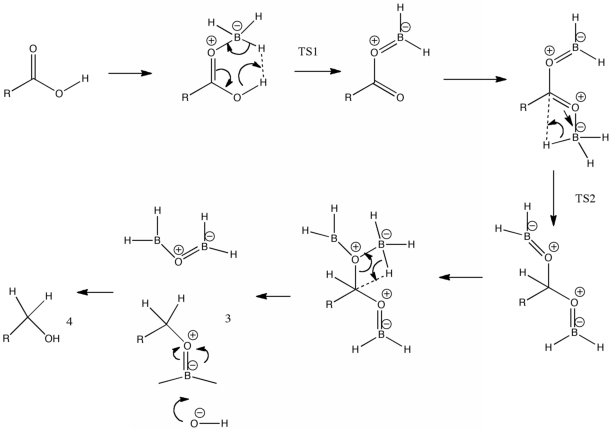
( ) leading to an acyloxyborane Note how this later species is rendered planar via conjugation, with electron flow being towards the B atom, away from the carbonyl group (these atoms are shown in orange ), rather than towards it (normally the case with carboxyl groups). This renders the carbonyl group more electron deficient than a carboxylic acid, and hence susceptible to hydride attack. A second borane now coordinates to the C=O group (via one of the lone pairs on the oxygen), and then transfers a hydride to the carbon via transition state 2 (( ). The product is then attacked by further borane, followed by Hydride transfer to the carbon (3), and final (work up) hydrolysis of the borate ester to the alcohol 4.
| -83.3 kcal/mol | Transition state 1; -58.2 kcal/mol |
|---|---|
| Intermediate for C=O reduction | Transition state 2 for C=O reduction |
| Product diol borate ester | |
Calculations done using a combination of AM1 (to locate initial transition states, about 20 sec) followed by B3LYP/6-31G(d) re-optimisation and calculation of the normal transition state mode (about 10 minutes).