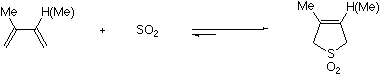
SULFUR DIOXIDE: Colourless gas with characteristic acrid odour. b.p. -10deg.C. Moderately soluble in water. To be used only in a fume cupboard. TOXIC BY INHALATION. SEVERE IRRITANT SKIN, EYES AND RESPIRATORY SYSTEM. Avoid breathing gas. O.E.L. 13 mg m-1.
Toxic effects The gas irritates all parts of the respiratory system. Extremely irritating to eyes.
Disposal Clear area of people. Allow to evaporate in a well ventilated area, preferably a fume cupboard.
ISOPRENE / 2,3-DIMETHYLBUTADIENE: Colourless gases; immiscible with water. TOXIC BY INHALATION. IRRITANT TO SKIN, EYES AND RESPIRATORY SYSTEM. Avoid breathing gases. O.E.L. not given.
Toxic effects. The gases irritate the eyes and the respiratory system.
Disposal. Clear area of people. Allow to evaporate in a well ventilated area, preferably a fume cupboard.
While the ability of the sulfonyl group to achieve inductive stabilisation of a carbanion has proved to be a highly effective and popular strategy for organic synthesis1, it is only recently that this chemistry has been used in cyclic sulfone systems2.
This experiment demonstrates the preparation of a prototypical building block by the cheletropic addition3 of a diene with sulfur dioxide.
The reaction to be carried out here4 is shown in the Scheme.
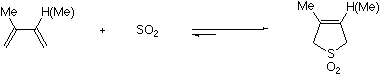
Scheme
In your write up, discuss the uses of these cyclic sulfones in synthesis.
Experimental
Guidelines for the Use of Pressure Bottles.
For high pressure reactions, metal autoclaves are generally available in laboratories, but it is frequently more convenient in small scale work (<= 100 ml) to use a thick walled glass bottle. The reaction mixture will not then be in contact with metal. (Vitreous enamel lined autoclaves are obtainable, but they are expensive and easily damaged).
i. Pressure bottles should not be more than half filled in order to leave ample room for expansion of the liquid phase.
ii. A good estimate of the pressure which will develop can be obtained from vapour pressure - temperature tables5. The relief valve in the cap of the bottle will burst when the internal pressure exceeds ca. 10 atmospheres.
iii. At the end of the experiment, the bottle must be allowed to cool to room temperature before the pressure is released and the cap is removed.
iv. The reaction system must be kept in a fume cupboard and behind a blast screen at all times whilst it is under pressure.
Procedure4.
A cooled (<= -20deg.C, use a cardice/acetone bath) glass pressure vessel is charged with isoprene or 2,3-dimethylbutadiene (5.1 g or 6.2 g respectively, 0.075 mol), liquefied sulfur dioxide (~10 ml), hydroquinone (0.17 g, 1.55 mmol) and methanol (4 ml). The vessel is sealed and heated slowly to in a water bath to a gentle boil (of the bath).
After 4 h at 85deg.C the bottle is allowed to cool, opened, the volatile components are evaporated under reduced pressure and the residue taken up in fresh methanol. The mixture is heated at reflux (if it is highly coloured, decolourising charcoal may be added at this point), the insoluble material filtered off and the solution allowed to cool when the product crystallises out.
Record the yield, m.p. and spectral data for the compound.
References.
1 T. Durst in 'Comprehensive Organic Chemistry', Eds. D.H.R. Barton and W.D. Ollis, Pergamon Press, Oxford, vol. 3, ch. 11.8 and 11.9, pp 171-213.
2 See inter alia H. Takayama, H. Suzuki, T. Nomoto and S. Yamada, Hererocycles, 1986, 24, 303; C.S.V. Horge-Frydrych, W.B. Motherwell and D.M. O'Shea, J. Chem. Soc. Chem. Commun., 1987, 1819.
3 I. Fleming, 'Frontier Orbitals and Organic Chemical Reactions', Wiley, London, 1976, p. 96; T.L. Gilchrist and R.C. Storr, 'Organic Reactions and Orbital Symmetry', 2nd. edn. Cambridge University Press, Cambridge, 1979, pp. 81, 159, 229.
4 H. Baker and J.A. Botema, Rec. Trav. Chim. Pays Bas, 1932, 51, 294; R.L. Frank and R.P. Seven, Org. Synth. Coll. Vol. III, p. 499; H. Staudinger and B. Ritzenthaler, Ber., 1935, 68, 455.
5 'Handbook of Chemistry and Physics', Chemical Rubber Company,Cleveland, Ohio, 66th Edn. 1985/6, p.D192 et seq. and references there cited.