
![]() EXPERIMENT 7:
EXPERIMENT 7:
CATALYTIC ASYMMETRIC DIHYDROXYLATION OF OLEFINS
To ![]() prepare
prepare ![]() an
an ![]() enantiomerically
enantiomerically ![]() enriched
enriched ![]() 1,2-diol
1,2-diol ![]() using
using ![]() Sharpless'
Sharpless' ![]() catalytic
catalytic ![]() asymmetric
asymmetric
dihydroxylation procedure, and to estimate the enantiomeric purity of the material you obtain.
Asymmetric catalysis; recrystallisation; measurement of optical rotations.
The need to prepare compounds such as drug candidates in enantiomerically pure form means that
the organic chemist needs at his/her disposal a wide range of small chiral organic molecules from
which to construct their target molecules. One source of these chiral starting materials (sometimes
called 'chirons') is nature itself. However, such a 'chiral pool' supply is necessarily limited - the
pool may not contain a particular type of starting material, or it may only be available as the
opposite enantiomer to the one needed.
Thus, chemists have begun to devise methods for the synthesis of ![]() both
both![]() enantiomers of useful
enantiomers of useful
small molecules from achiral starting materials.![]() 1
1![]() This
This ![]() can
can ![]() be
be ![]() achieved
achieved ![]() through
through ![]() temporary
temporary
attachment of a chiral auxiliary to the achiral material (the synthesis is then diastereoselective,
rather than enantioselective); use of chiral reagents; or the use of chiral catalysts. The latter
approach,2![]() though the less well developed of the three, is potentially the most interesting, since a
though the less well developed of the three, is potentially the most interesting, since a
small amount of chiral catalyst can produce large amounts of enantiomerically enriched product.
One of the pioneers of this field has been Professor K Barry Sharpless of the Scripps Research
Institute. The eponymous epoxidation of allylic alcohols![]() 3
3![]() was the first practical and reliable
was the first practical and reliable
catalytic asymmetric reaction, and more recently he has turned his attention to the asymmetric
osmium mediated dihydroxylation![]() 4
4![]() (see scheme) and aminohydroxylation
(see scheme) and aminohydroxylation![]() 5
5![]() of olefins, using
of olefins, using
modified cinchona alkaloids as chiral ligands for osmium.
![]() Chemistry, Imperial
Chemistry, Imperial![]() College
College
![]() Advanced Practical Organic
Advanced Practical Organic![]() Chemistry
Chemistry
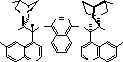
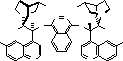
![]() H
H
![]() H
H
![]() H
H
![]() N
N
dihydroquinine
![]() H
H
![]() N
N
dihydroquinidine
![]() M
M
![]() R
R![]() S
S
R![]() L
L
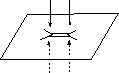
![]() α
α![]() -face
-face
predicting the
sense of asymmetric
induction in AD reactions
![]() 4
4
![]() +
+
Ligand
![]() L
L
![]() O
O
O
![]() 2
2![]() PHAL
PHAL