TETRAHYDROFURAN: Colourless volatile liquid with ethereal odour; b.p. 66deg.C; miscible with water. Liable to form explosive peroxides on exposure to light/air. Peroxides removed by treatment with aqueous sodium metabisulfite. HARMFUL VAPOUR. FORMS EXPLOSIVE PEROXIDES. EXTREMELY FLAMMABLE. Avoid breathing vapour and eye contact. O.E.L. 590 mg m-3.
Toxic effects. The vapour irritates eyes and respiratory system; high concentrations have narcotic effect. Absorption or ingestion may cause liver damage.
Hazardous. Explosive peroxides formed on exposure to air/light. NaOH/KOH can cause explosion with peroxided material.
Fire hazard. Flash point -17deg.C; ignition temp. 321deg.C; extinguish fire with CO2.
Spillage. Clear area, shut off all sources of ignition. Mop up with plenty of water and run to disposal waste. Organise effective ventilation and evaporate remaining liquid.
DIBUTYL ETHER: Colourless liquid; b.p. 142deg.C; immiscible with water. Liable to form explosive peroxides on exposure to light/air. Peroxides removed by treatment with aqueous sodium metabisulfite. HARMFUL VAPOUR. FORMS EXPLOSIVE PEROXIDES. EXTREMELY FLAMMABLE. Avoid breathing vapour. avoid skin/eye contact. O.E.L. not given.
Toxic effects. The vapour is somewhat irritating to the respiratory system. The liquid irritates the eyes and is hazardous by skin absorption.
Hazardous. Peroxide formation can result in subsequent explosion. Powerful oxidants can cause explosion.
Fire Hazard. Flash point 25deg.C, ignition temp. 194deg.C; extinguish fire with CO2.
Spillage. Wear face shield, goggles and gloves. Absorb bulk quantities on sand, shovel disposal into containers and remove to a chemical disposal skip. Wash site of spillage with plenty of water and detergent.
CHROMIUM HEXACARBONYL: Colourless volatile crystals, m.p. 150deg.C (dec); immiscible with water. HIGHLY TOXIC BY INGESTION OR INHALATION. Avoid skin/eye contact. Avoid breathing vapour. O.E.L. 0.24 mg m-3 [assessed as Ni(CO)4]
Toxic effects. Highly toxic by inhalation. Avoid all exposure. Handle only in an efficient fume cupboard.
Spillage . Do not attempt to deal with a spillage. Call a Demonstrator or Technician disposalimmediately.
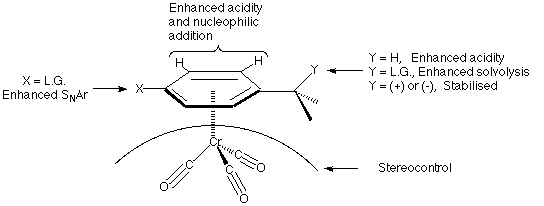
The exploitation of the modification of reactivity of an organic molecule by complexation to a metal is one of the major areas of development in organic synthesis in recent times1. One particular area is the study of the enhanced reactivity of arenes upon complexation by Group VI metal and manganese carbonyls. Among these by far the most studied are the arenechromiumtricarbonyl complexes2. The effect of the metal moiety on the arene ring is an apparent electron withdrawal from the [[pi]]-system and this manifests itself in a variety of ways as shown below:--

It is important for the ease of use of such complexes that they should be readily prepared and efficiently decomplexed. This experiment demonstrates the most convenient method of preparation via the use of the Strohmeier apparatus3 and a simple vacuum line/nitrogen manifold (see Appendix). The complexes are synthesised by the direct reaction between the arene and chromium hexacarbonyl in an ether solvent mixture (Bu2O-THF 10-1)4.
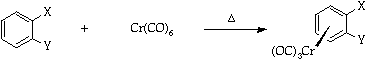
The need for the Schlenk line arises from the fact that the intermediates generated during the synthesis, the coordinatively unsaturated chromium carbonyl species, are very oxygen sensitive and rigorously anaerobic conditions are essential. Once formed the arene complexes are air stable in the solid state and can be handled without difficulty by conventional techniques.
The Strohmeier apparatus is, in effect, an inverted condenser. This is a convenient way of dealing with the problem of the volatility of the metal carbonyl which tends to condense above the level of the solvent in a normal condenser and block it. In the Strohmeier apparatus, any hexacarbonyl condensing ahead of the solvent is washed back into the reaction vessel via the syphon.
Experimental
A mixture of di-n-butyl ether (purified by distillation from sodium-benzophenone5, (60ml), THF (purified by distillation from sodium-benzophenone5 (6ml), the arene [one example from fluorobenzene, chlorobenzene, anisole, or other as suggested by a DAW; quantity: 1 equivalent (for valuable substrates) or 10ml (for readily available substrates)] and chromium hexacarbonyl (1.0g) are placed, together with a magnetic stirrer bar, in a 100ml round bottomed flask and attached to the Strohmeier apparatus. The assembly is connected to the vacuum line/nitrogen manifold using semipressure tubing and a magnetic stirrer and a heating bath on a lab jack, placed beneath the round bottomed flask.
Check that all joints are sealed then evacuate the apparatus by carefully turning the 3-way tap on the frame (see APPENDIX for the vacuum line/nitrogen manifold system). As soon as the solvent begins to boil, let in the dry, oxygen free nitrogen via the 3-way tap. Repeat this cycle nine more times to ensure that the system is completely anaerobic. With the system maintained under a slight positive pressure of nitrogen by the manifold commence stirring and heating the contents of the flask. Maintain the solution at a steady reflux for ~ 24h.
At the end of the reaction, the flask is cooled then detached from the Strohmeier apparatus. The solution is chromatographed over a short (~-5cm) column of silica gel 60 using ether to complete the elution of the yellow/orange complex. Evaporation of the solvents, using a cardice Büchi evaporator with minimal bath heating, yields the product.
Record the m.p. and record and interpret the i.r. and n.m.r. spectra. In your write up, comment on the synthetic uses of these complexes.
References and Notes
1. For broad surveys see: "Comprehensive Organometallic Chemistry" Eds G.Wilkinson, F.G.A.Stone and E.W.Abel, Pergamon Press, Oxford, 1982, Vols 7 and 8; S.G.Davies, "Organotransitionmetal Chemistry: Applications to Organic Synthesis" Pergamon Press, Oxford, 1982; "Organometallics in Organic Synthesis" by E.-I. Negishi, Wiley-Interscience, New York, 1980.
2. M.F.Semmelhack, G.R.Clark, J.L.Garcia J.J.Harrison, Y.Thebtaranonth, W.Wulff, and A.Yamashita, Tetrahedron, 1981, 37, 3957; G.Jaouen, Ann.N.Y.Acad.Sci., 1977, 295, 59; M.F.Semmelhack, ibid., p. 36; D.A. Marsden, Phil. Trans. Roy. Soc. Lond., 1988, A 326, 595.
3. W.Strohmeier, Chem.Ber., 1961, 94, 2490.
4. This solvent mixture has been determined empirically to give the optimum reaction temperature and a good backwash of the volatilised chromium hexacarbonyl. See C.A.L. Mahaffy and P.L.Pauson, Inorg. Syntheses, 1979, 19, 154.
5. A special communal still for the purification of this solvent is set up in fume cupboard No. 8. It is essential that you consult a Demonstrator or a Technician before you use it.
APPENDIX
The line is simple to use, but note carefully the following points:-
1. Study the frame before you use it and make sure that you understand its operation.
2. The nitrogen purifying column is chromium(II) adsorbed on silica gel. It must NEVER be opened to atmosphere. If this occurred it would get dangerously hot.
3. In order to maintain a positive pressure of nitrogen in the system, the pressure should be adjusted to give a gentle flow through the mercury bubbler.
4. Turn taps slowly and carefully. Make sure that you understand the consequences before you turn any tap.
5. Always top up the liquid nitrogen trap in the vacuum line before starting to use the frame. It is essential that you protect the vacuum pump in this way, we cannot afford replacements.
HAZARD WARNINGS
CHROMIUM HEXACARBONYL: A colourless crystalline solid with a high vapour pressure. HIGHLY TOXIC BY INGESTION OR INHALATION. All open manipulation must be carried out in a fume cupboard. In the event of a spillage, call a Demonstrator or Technician IMMEDIATELY.