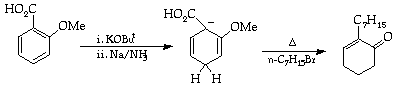
AMMONIA(gas): Colourless gas with characteristic odour, b.p. -33deg.C. Miscible with water.
TOXIC BY INHALATION. IRRITANT TO SKIN EYES AND RESPIRATORY SYSTEM. FLAMMABLE. Avoid breathing gas and skin contact. O.E.L. 17 mg m-1.
Toxic effects. The gas irritates all parts of the respiratory system. Extremely irritating to eyes.
Hazardous. Reacts vigorously with oxidising agents. Mixtures with air can be explosive reactions
Fire hazard. Explosive limits 16-25%; ignition temp. 651deg.C.
Disposal. Clear area of people. Allow to evaporate in a well ventilated area, preferably a fume cupboard.
1,2-DICHLOROETHANE: Colourless liquid with a chloroform like odour, b.p. 84deg.C. Immiscible with water. HARMFUL VAPOUR. IRRITANT TO SKIN EYES AND RESPIRATORY SYSTEM. HIGHLY FLAMMABLE. Avoid breathing vapour and skin/eye contact. O.E.L. 40 mg m-1.
Toxic effects. In high concentrations the vapour irritates the eyes and respiratory system; it may also cause drowsiness, headache, vomiting and mental confusion. The liquid can cause serious damage to the eyes. Poisonous by mouth. Continued exposure may result in damage to eyes and liver. Repeated skin contact may cause dermatitis.
Hazardous. Reacts vigorously with reactive metals such as K, Na, and Al powder.
Fire hazard. Flash point 13deg.C, explosive limits 6.2-15.9%; ignition temp. 413deg.C; extinguish fire with CO2.
Spillage. Wear face shield, goggles and gloves. Absorb bulk quantities on sand, shovel into containers and remove to a chemical disposal skip. Wash site of spillage with plenty of water and detergent.
TETRAHYDROFURAN: Colourless volatile liquid with ethereal odour; b.p. 66deg.C; miscible with water. Liable to form explosive peroxides on exposure to light/air. Peroxides removed by treatment with aqueous sodium metabisulfite. HARMFUL VAPOUR. FORMS EXPLOSIVE PEROXIDES. EXTREMELY FLAMMABLE. Avoid breathing vapour and eye contact. O.E.L. 590 mg m-3.
Toxic effects. The vapour irritates eyes and respiratory system; high concentrations have narcotic effect. Absorption or ingestion may cause liver damage.
Hazardous. Explosive peroxides formed on exposure to air/light. NaOH/KOH can cause reactions explosion with peroxided material.
Fire hazard. Flash point -17deg.C; ignition temp. 321deg.C; extinguish fire with CO2.
Spillage Clear area, shut off all sources of ignition. Mop up with plenty of water and run to waste. Organise effective ventilation and evaporate remaining liquid.
SODIUM: Supplied as soft, silvery white sticks, normally coated with grey-white oxide/carbonate. Reacts vigorously with water generating H2 which may ignite. DANGEROUS ON CONTACT WITH WATER. CAUSES BURNS. Avoid eye/skin contact.
Toxic effects. Contact with moist skin causes caustic and thermal burns.
Hazardous. Very reactive, explosively so with many acids. Dispersions in inert solvents pyrophoric. Reacts violently with many organic halocompounds.
Spillage . Clear area, shut off all sources of ignition. Wear face shield goggles and gloves. If contained, destroy cautiously with isopropanol. If scattered, cover with solid sodium carbonate, shovel into buckets and remove to safe place for destruction with isopropanol.
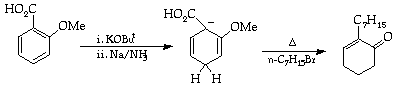
Preliminaries
a). Tetrahydrofuran should be dried and made oxygen free by boiling over sodium/benzophenone ketyl under nitrogen and distilling just before use.
b). Set up the apparatus in the fume cupboard, as shown in the Figure (p. 2), making sure that the stand and clamps are secure. For optimum yields it is important to use dry apparatus and to exclude moisture. Always use a fresh rubber tube to lead the ammonia vapour from the cylinder to the reaction flask. Tubing left connected to the cylinder may contain moisture.
c). In planning this experiment, check in advance with the Technician that the apparatus is available when you want it and make sure that you have sufficient time to complete the reactions (~ 3-4hrs).
Experimental
Charge the three-necked, 500 ml, round-bottomed flask with o-anisic acid (7.6 g, 0.05 mol), tetrahydrofuran (50 ml) and (CARE) potassium t-butoxide (5.6 g, 0.05 mol). Connect the inlet tube to the ammonia cylinder. Fill the acetone-dry ice condenser and the cooling bath with solid carbon dioxide pellets and then carefully add acetone to the bath and condenser to obtain the operating temperature of -78deg.C. Carefully open the liquid ammonia cylinder, whereupon liquid ammonia will condense from the low temperature condenser. In this manner distil in 200 ml of ammonia.
Figure
The resulting thick white suspension of the ammonium salt of the acid is stirred mechanically while small pieces of freshly cut sodium, washed sequentially with toluene and ether, are added. The suspension dissolves to give a clear yellow solution, which on introduction of more sodium changes to the characteristic deep blue colour of excess sodium.
When the colour persists add, all in one portion, a mixture of 1-bromoheptane (10.75 g, 0.06 mol) and 1,2-dibromoethane (0.5 ml, 1.2 mmol). The blue colour is discharged immediately, leaving a yellow solution. Remove the acetone-dry ice cooling bath and the condenser and allow the ammonia to evaporate under a gentle stream of nitrogen.3
Dilute the residue with water (350 ml) (CAUTION) and wash the resulting aqueous solution with dichloromethane (3 x 20 ml). Acidify the aqueous extract with cold concentrated hydrochloric acid and reextract with portions of 1,2-dichloroethane (5 x 20 ml). Place the combined 1,2-dichloroethane extracts in a 250 ml, one-necked round bottomed flask bearing a reflux condenser; add water (25 ml), concentrated hydrochloric acid (25 ml) and hydroquinone (150 mg), and heat the mixture at reflux under nitrogen for 30 min.
After cooling, separate the layers and wash the organic phase with aqueous potassium carbonate solution (0.5M, 30 ml) (CAUTION, CO2 evolution). Dry the organic layer over anhydrous potassium carbonate, filter, and remove the solvent using the rotary evaporator. Examine the crude organic product by i.r. and n.m.r. spectroscopy.
Distil the product through a bulb to bulb distillation apparatus (Kügelrohr) connected to the turbopump. Collect a centre cut boiling at 100-104deg.C / 0.02 mm Hg. You must consult a demonstrator or technician before attempting to use this apparatus. Record the boiling point, yield, i.r. and n.m.r. spectra of the purified product.
In your report, comment on the mechanisms involved in the above transformation, and on the utility of the Birch reduction for organic synthesis.
References
1. a) A.J. Birch, Quart. Rev., 1950, 4, 69; b) A.J. Birch and H. Smith, ibid., 1958, 12, 17. c) H. Smith, "Organic Reactions in Liquid Ammonia, Chemistry in Non-aqueous Ionizing Solvents", Vol. 1, part 2, John Wiley, New York, 1963; d) M. Smith in "Reduction", Ed. R.L. Augustine, Marcel Dekker, New York, 1968, pp 95-170; e) W. Reusch, ibid., pp 186-194.
2. For more recent applications of this reductive alkylation approach see: a) J.M. Hook, L.N. Mander and R. Urech, Synthesis, 1979, 374; b) L.N. Mander, and R.J. Hamilton, Tetrahedron Lett., 1981, 22, 4115; c) J.M. Hook, L.N. Mander and M. Woolias, ibid., 1982, 23, 1095.
3. Because of the potential hazard to contractors working adjacent to the duct outlet, the rate of evaporation of ammonia must not exceed 170 g h-1.