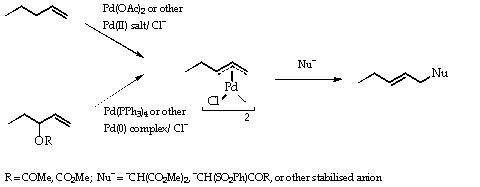
PHENYLACETYLENE: Colourless liquid, b.p. 142deg.C. Immiscible with water.
FLAMMABLE. HARMFUL VAPOUR. SKIN/EYE IRRITANT. Avoid breathing vapour and skin contact. O.E.L. not assigned
Toxic effects. Harmful by ingestion in quantity. May irritate skin and eyes.
Hazardous Can react vigorously with oxidising agents.
Fire hazard. Flash point 31deg.C; extinguish fire with CO2.
Spillage. Wear face shield, goggles and gloves. Absorb bulk quantities on sand, shovel into containers and remove to a chemical disposal skip. Wash site of spillage with plenty of water and detergent.
1-BROMO-4-NITROBENZENE: Colourless crystals, m.p. 127deg.C. Immiscible with water. VERY TOXIC. SKIN/EYE IRRITANT. Avoid breathing vapour and skin contact. O.E.L. not assigned
Toxic effects. Very harmful by ingestion in quantity. Irritant of skin and eyes. Danger of cumulative effects.
Hazardous. May react with oxidising agents.
Spillage. Wear face shield, goggles and gloves. Absorb bulk quantities on sand, shovel into containers and remove to a chemical disposal skip. Wash site of spillage with plenty of water and detergent.
TRIETHYLAMINE: Colourless liquid, ammoniacal odour, b.p. 89deg.C. Miscible with water. HIGHLY FLAMMABLE. HARMFUL VAPOUR. STRONG SKIN/EYE IRRITANT. Avoid breathing vapour and skin contact. O.E.L. 40 mg m-1.
Toxic effects. Harmful by ingestion causing internal irritation and damage. Extremely irritating to skin and eyes and respiratory system.
Hazardous. Reacts vigorously with oxidising agents
Spillage. Wear face shield, goggles and gloves. Absorb bulk quantities on sand, shovel into containers and remove to a chemical disposal skip. Wash site of spillage with plenty of water.
Transition metal mediated reactions are becoming increasingly important in organic synthesis as organic chemists become familiar with the myriad of organometallic mechanisms involved in these processes.1
Palladium is by far the most developed and used as far as application to organic synthesis is concerned2 and indeed homogeneous palladium catalysts are used in the commercial production of acetaldehyde, cinnamates and other products. Palladium undergoes many reactions with organic systems and, apart from the well known hydrogenation and oxidation (Wacker and other2) processes, it acts as a catalyst for many key C--C bond forming reactions.
Three areas are particularly important, the [[pi]]-allyl- ([[eta]]3-allyl)-palladium (Scheme 1), the cross coupling (Scheme 2) reactions and the Heck reactions (Scheme 3). The first of these was extensively developed by Trost,3 the second by Kumada4 and later by Stille5, Suzuki6 and others and the last by Heck himself.2 As indicated in the Schemes, the catalyst is frequently a Pd(0) species yet the added material may be a Pd(II) salt. In these cases, the Pd(0) is generated in situ by reduction with the organometal reagent or by solvent.
Scheme 1
Scheme 2
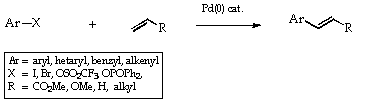
Scheme 3
Additionally, carbonylation (with CO) can frequently be included in the reaction sequence to generate acylated products2, a detailed example is given in Scheme 47.
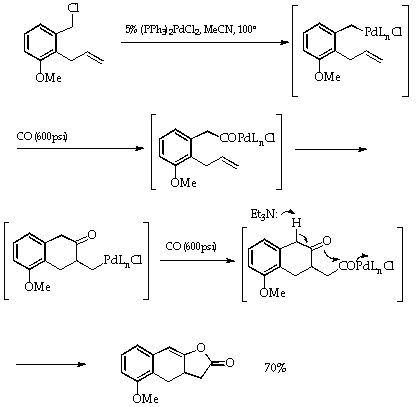
Scheme 4
A full account of this chemistry, including the generally accepted mechanisms, can be culled from the references cited (1-7). It is important to note that the ready oxidative addition of the aryl (and alkenyl) halides and triflates to the Pd(0) species reverses the conventional concepts of reactivity in aryl systems. In nucleophilic displacement processes, as normally encountered, the alkyl halides are the more reactive. In the palladium catalysed reactions, the alkyl halides are the unreactive species.
In this experiment, taken from ref 2, p. 300 and repeated below, 4-nitrobromobenzene is cross coupled with phenylethyne, as its presumed triethylamine salt, to give the mixed diarylalkyne (Scheme 5). The product is conveniently purified by vacuum sublimation, a clean and much underused technique.
Scheme 5
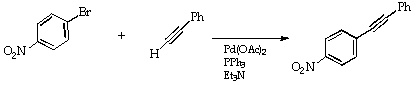
Experimental
In a 100 ml 3-necked flask are placed 4-bromonitrobenzene (2.02 g 10 mmol), phenylethyne (1.53 g, 1.65 ml, 1.5 equiv), palladium acetate (2.8 mg, 0.12 mol%, triphenylphosphine (6.6 mg, 0.25 mol%), triethylamine (20 ml) and a magnetic stirrer bar. The flask is fitted with a condenser, attached to a N2 supply with a pressure relief bubbler, and the two remaining ports stoppered after the flask has been purged with N2. The solution is stirred and heated gently to 100deg.C for 75 min. The exothermic reaction will initially cause vigorous boiling, but this will subside as the process nears completion.
The reaction mixture is cooled to room temperature and 2M HCl (42 ml) added. The insoluble product is collected by filtration and dried in a drying pistol overnight. The product is then sublimed at ~115deg.c at 2-3 mm Hg. The sublimate is dissolveded in ~70% ethanol/water and left in a fridge overnight to complete crystallisation. The crystals are finally collected and dried in vacuo.
Record the yield, m.p., ir and nmr spectra and interpret the latter. In your write up discuss the probable mechanism of the reaction.
References
1. For an excellent general account, see 'Principles and Applications of Organotransition Metal Chemistry' by J.P. Collman, L.S. Hegedus, J.R. Norton and R.G. Finke, University Science Books/OUP, 1987.
2. For a comprehensive account of palladium in organic synthesis, including full experimental details, see 'Palladium Reagents in Organic Synthesis' by R.F. Heck, Academic Press, 1985.
3. B.M. Trost and T.R. Verhoeven, in 'Comprehensive Organometallic Chemistry', eds G. Wilkinson, F.G.A. Stone and E.W. Abel, Pergamon Press, 1982, vol. 8, pp. 799-938.
4. T. Hayashi, M. Konishi and M. Kumada, Tetrahedron Lett., 1979, 1871; and references there cited.
5. J.K. Stille, Angew. Chem. Int. Ed. Engl., 1986, 25, 508.
6. N. Miyaura, K. Yamada, H. Suginome and A. Suzuki, J. Am. Chem. Soc., 1985, 107, 972.
7. For this recent example see G. Wu, I. Shimoyama and E.I. Negishi, J. Org. Chem., 1991, 56, 6506.