

UV LIGHT. Uv light is extremely dangerous to the eyes. When carrying out photolyses, always wear tinted uv glasses (obtainable from the Stores) and never look directly at the lamp with unprotected eyes. Keep the lamp shielded from other persons in the laboratory and carry out the photolysis only in a fume cupboard
On irradiation, compounds absorb light and undergo electronic excitation provided that the light energy corresponds to the difference between ground state and excited state energy levels. Most molecules have a singlet ground state and on photolysis are converted initially into the first excited singlet level. The energy gained can be dissipated in a number of ways. it may be re-emitted (fluorescence), or dissipated by conversion to heat as increased vibrational motion.
Alternatively, the first excited singlet state can be converted into the lower energy first excited triplet state (intersystem crossing) and thence to the ground state singlet level by either a 'forbidden' i.e. low probability, radiative process (phosphorescence) or by non-radiative processes.
Conversion of the first excited state triplet to the ground state singlet of one molecule can excite a second (different) molecule from its ground state singlet to its first excited state triplet provided that the energy levels are matched (quenching and sensitisation respectively).
In this way, the radiant energy absorbed by a sensitiser can be passed on to a more susceptible compound which then undergoes reaction. The sensitiser, having returned to its ground state is available for re-excitation. It therefore acts as a photochemical catalyst.
The geometry, electron distribution and bond strengths of molecules in excited states are different from the ground state. This gives rise to novel chemical reactions. If the light absorbed is of sufficient energy, a molecule is converted into a high vibrational level of the first excited singlet and thus homolysis takes place.
The efficiency of a photochemical reaction is measured by the quantum yield: the number of molecules of reactant consumed or product formed per photon absorbed.
Photolysis of santonin.2
Cross conjugated dienones, such as santonin, undergo unusual reactions on irradiation (see Scheme, p. 3) and are some of the most studied systems. Santonin is the classic example.
The products formed from santonin vary considerably with the choice of solvent and temperature2 and careful experimentation is needed to achieve the desired product. Above all, over photolysis must be avoided in order to isolate the lumisantonin. The course of the reaction can be followed conveniently by tlc and ir spectroscopy and with more precision, but requiring more care, by u.v.spectroscopy.
The various references given, and others cited in them, should be carefully studied before commencing this experiment. It is the objective of this experiment to isolate lumisantonin. If the reaction is over irradiated and one of the later photoproducts is produced, this is an acceptable result but it will carry fewer marks.
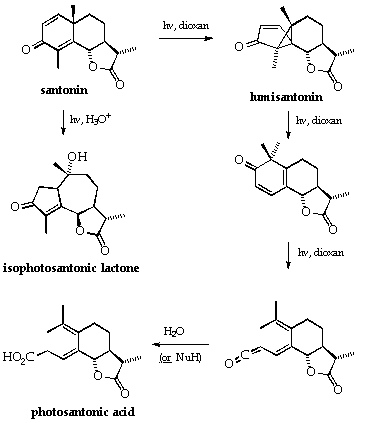
Scheme
Preliminaries
1. Establish a tlc system for santonin which gives an RF of ~0.5 on alumina plates.
2. Ensure that the dioxan purification still is on and that the benzophenone indicator is blue, ready for the supply of pure material.
3. Purify petroleum ether, b.p. 40-60deg.C by distillation.
4. Purify toluene by distillation from calcium hydride.
Experimental
The photochemical reactor is very fragile and very expensive. Treat it with the utmost care and never subject it to undue stress.
1. Rubber/plastic tubing to be attached should always be expanded by soaking the end in chloroform (for rubber) or warming it with a hair dryer (for plastic) before assembly. Tubing must always be removed by carefully cutting it off with a scalpel, never attempt to pull it off.
2. Never over tighten the clamps holding the apparatus.
Assemble the reactor as shown in the figure.
Figure
Dissolve santonin (1.0 g) in anhydrous dioxan (100 ml) and place in the photolysis apparatus (Figure). Pass a stream of purified nitrogen, presaturated by passing it through a dioxan filled bubbler, through the sintered disk in order to agitate the solution and maintain an anaerobic solution. With the nitrogen flowing, by means of a long needle syringe passed through the septum, remove a small aliquot of the solution to a sample tube and assay the mixture qualitatively by tlc and ir spectroscopy (for the ir, the solvent must be removed by gently blowing on the surface of the solution with nitrogen from a Pasteur pipette before the spectrum can be taken).
Ensure that water is flowing through the cooling jacket. Surround the apparatus completely with aluminium foil as a uv safety measure and to ensure maximum irradiation of the reactant. Switch on the mercury arc lamp. At 15 min intervals, sample the solution as before and assay the mixture qualitatively by tlc and ir spectroscopy. Continue until the starting material is just consumed, normally <= 1 hour. For reasons obvious from the Scheme do not over irradiate.
When the reaction is complete, switch off the water supply and the lamp, allow the latter to cool down and remove it from the quartz lamp-well.
Carefully withdraw the quartz lamp-well from the rest of the apparatus, in the process rinsing it into the reactor well with toluene. Transfer the reaction solution to a round bottomed flask, again rinsing it through with toluene. Remove the solvents (Rotavapor), keeping the solution below 35deg.C.
Prepare a chromatographic column using a slurry of BDH neutral Brockmann Grade III alumina (~30 g) in petroleum ether (b.p. 40-60deg.C). Apply the photolysate in a small volume of petroleum ether (a little toluene may be necessary to effect solution) and develop the column with an increasing gradient of toluene in petroleum ether (~100 ml each of 2%, 5%, 10%, 25% etc). Collect 25 ml fractions, and examine each by tlc (evaporation of the solvent may be necessary if the concentration is too low). Combine like fractions and evaporate the solvent (Rotavapor) and recrystallise the residue from acetone/hexane (or other suitable solvent) to constant m.p.
Record the yield of the purified material and record and interpret the ir, nmr and uv spectra and hence determine which of the photoproducts was obtained. Discuss the mechanisms of the Scheme.
References
1. For a brief general account, see: Jerry March, 'Advanced Organic Chemistry', 4th edn, John Wiley, 1992, p 231. March refers to many modern texts on organic photochemistry. Dependent upon availability, these may be usefully consulted for more detailed discourses of both the fundamental principles and applications in synthesis.
2. D.H.R. Barton, Helv. Chim. Acta, 1959, 42, 2604; D.I. Schuster and A.C. Fabian, Tetrahedron Lett., 1966, 4093; D.C. Neckers, 'Mechanistic Organic Photochemistry', Reinhold, New York, 1967, pp 217-224.