OZONE: A toxic gas which forms explosive ozonides with many olefins. TOXIC BY INHALATION. SKIN/EYE IRRITANT. EXPLOSIVE WITH REDUCING AGENTS. Avoid inhalation and skin/ eye contact. O.E.L. 0.2 mg m-3.
Toxic effects. Gas strongly irritates the upper respiratory tract and may cause headache. High concentrations have caused death by lung congestion in animals.
Hazardous. Solid and liquid ozone are highly explosive; forms explosive peroxides with alkenes and other organic molecules. Explodes with many reducing agents; Br2, HBr, N2O4.
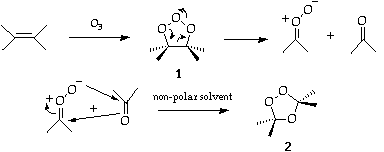
Ozone readily attacks ethylenic linkages and from the products, carbonyl compounds can be obtained1. The process results in separation of the carbon atom originally joined by the double bond. The identities and yields of carbonyl products provide information on the positions of the double bonds in the olefin.Hence ozonolysis is frequently used in structure determination as well as for synthetic purposes.
The work of Criegee2 indicates that the preferred mode of addition to most olefins is via a molozonide (1). In some cases these can be isolated; reduction at low temperatures can then be made to produce glycols. In general however, the molozonide (1) rapidly decomposes to carbonyl and peroxy products. These rapidly recombine in non-polar solvents to give an ozonide (2).

Occasionally, a polymer results or the carbonyl oxide may react with solvent. In addition, the carbonyl oxide can be captured by (excess) added carbonyl compound and the reaction diverted to a different ozonide.
Hydrolysis or (preferably) reduction of the the ozonide (2) liberates the two carbonyl compounds.
In the cases where an aldehyde would be formed, the equivalent of peroxide generated by hydrolysis produces carboxylic acid instead of aldehyde. It is therefore preferable to use the reductive work up to reduce the peroxy link. As reducing agents, Zn/AcOH, Me2S, Ph3P, Me3P, and (NC)2C=C(CN)2 have all been used (see Scheme 1). Catalytic hydrogenation is hazardous and not recommended.
Scheme 1
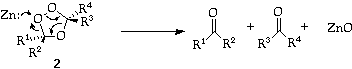
Conversely, treatment of the ozonide with peracids gives ketones and carboxylic acids directly.
Ozonisation is effected by passing a stream of dry ozonised oxygen (1-15% O3) through through a solution of the olefin in a suitable solvent (AcOH, CCl4, CHCl3, hexane, EtOAc are all used) at or below room temperature until the ozone is no longer rapidly absorbed. Over ozonisation will cause general oxidation and decomposition.
Ozonolysis of 2,4,4-Trimethylpent-1-ene
General
Purify a sample of the olefin by distillation before use. Assay the pure product by n.m.r. spectroscopy. The overall reaction is:--
Me3CCH2C(Me)=CH2 [[arrowhorizex]][[arrowhorizex]][[arrowhorizex]][[arrowhorizex]][[arrowhorizex]][arrowhorizex]]-> Me3CCH2COMe + CH2O
Formaldehyde is isolated as the dimedone derivative and the neopentyl methyl ketone as its 2,4-dinitrophenylhydrazone.
The Ozone Generator.
The commercial Triogen generator produces 3.1g/hr of ozone at an oxygen flow rate of 1 l/min. Do not deviate from this flow rate. The O3/O2 mixture must be passed through a reversed Drechsel bottle, placed between the generator and the reactor vessel, to prevent suck-back into the generator. Similarly, after the reactor, the flow should be passed through an empty Drechsel bottle, to catch entrained liquid, and vented high in the fume cupboard. All connections should be made with pvc tubing.
Method
The equipment must be set up in fume cupboard No. C51B.
Weigh accurately ~ 3.5 g (0.03125 mole) of the pure olefin into a large Drechsel bottle containing glacial acetic acid (100 ml). Wash in the last traces with a little acetic acid. Immerse the reactor up to the neck in an ice/water bath and connect into the gas flow system. Turn on the oxygen and adjust the flow rate (to 1 l/min) before switching on the generator. When everything is ready, get the set-up checked by a Demonstrator or Technician.
Finally, switch on the generator and pass the O3/O2 mixture for 30 min (0.03125 mole of O3). Switch off the generator and allow the O2 flow to purge the system for a few minutes. Switch off the gas and disconnect the reactor.
Pour the contents of the reactor into a 500 ml round bottom flask containing zinc dust (5 g) and wash the reactor with a little water. Add the washings to the r.b. flask and set this up for steam distillation (if you are unfamiliar with the technique, ask the Demonstrator or Technician for details). Steam distil as rapidly as possible and continue until the distillate contains insignificant amounts of carbonyl compound as judged by a 2,4-dinitrophenylhydrazine test.
Cool the distillate in ice-water, add 4-5 drops phenolphthalein and neutralise with 40% sodium hydroxide. As the solution becomes permanently pink, add acetic acid dropwise until the colour disappears. Add a saturated solution of dimedone (5,5-dimethylcyclohexane-1,3-dione) (4.5 g) in 50% aqueous ethanol. After 30 - 60 min., collect the precipitated derivative, wash it with 20% aqueous ethanol and recrystallise it from ethanol - water. Dry the product in a drying pistol and record the yield, m.p., n.m.r. and i.r. spectra.
Steam distil the filtrate from the dimedone derivative until all the ketone has come over (2,4-DNP test). The distillate is treated with 2,4-DNP reagent (from 6.2 g 2,4-DNPH3) and the precipitated derivative filtered off, washed with 30% aqueous ethanol and recrystallised from methanol. Record the yield, m.p., n.m.r. and i.r. spectra.
Ozonolysis of Oct-1-ene or cis/trans-Oct-2-ene
General
Purify a sample of the olefin by distillation before use. Assay the pure product by n.m.r. spectroscopy. The overall reactions are:--
Me(CH2)5CH=CH2 = Me(CH2)5CHO + CH2O
or
Me(CH2)4CH=CHMe = Me(CH2)4CHO + MeCHO
Here, both products are isolated as their 2,4-DNP derivatives.
Ozonolysis is carried out as above at the 0.0325 molar scale. After the first steam distillation, the distillate is not neutralised with sodium hydroxide, but treated directly with 2,4-dinitrophenylhydrazine in ethanolic mineral acid3. After 30 - 60 min, the mixture of derivatives is filtered off, dried and separated by flash chromatography (see Technique 2) eluting with dichloromethane - petroleum ether b.p. 40-60deg.C. Recrystallise each pure derivative from methanol and record the yield, m.p., and n.m.r. and i.r. spectra of each.
References
1. L.F. Fieser and M. Fieser, 'Reagents for Organic Synthesis', John Wiley, New York, vols, 1-15; P.S. Bailey, Chem. Rev., 1958, 58, 925; P.R. Story, et al., J. Am. Chem. Soc., 1965, 87, 737.
2. R. Criegee, Angew. Chem. Int. Ed. Engl., 1975, 14, 745.
3. a) A.I. Vogel, 'Practical Organic Chemistry' 4th Edn, Longmans, London, 1978, p. 1112; b) L.M. Harwood and C.J. Moody 'Experimental Organic Chemistry', Blackwell, 1989, p.260.