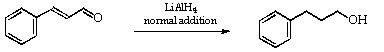
LITHIUM ALUMINIUM HYDRIDE. A white microcrystalline powder and lumps; decomposes above 125deg.C; reacts rapidly with water with evolution of hydrogen. EXPLOSIVE H2 LIBERATED BY WATER. Prevent skin, eye and clothing contact.
Toxic effectsReaction with water produces LiOH which is corrosive to skin and eyes.
Hazardous. May ignite when ground with a pestle. Reacts vigorously on solution if solvents not rigorously dried. See special warning below.
Fire hazard. Arises from contact with small quantities of water. Extinguish with sand, not CO2.
Spillage Clear area, shut off all sources of ignition. Wear face shield goggles and gloves. If contained, destroy cautiously with isopropanol. If scattered, cover with solid sodium carbonate, shovel into buckets and remove to safe place for destruction with isopropanol.
TETRAHYDROFURAN: Colourless volatile liquid with ethereal odour; b.p. 66deg.C; miscible with water. Liable to form explosive peroxides on exposure to light/air. Peroxides removed by treatment with aqueous sodium metabisulfite. HARMFUL VAPOUR. FORMS EXPLOSIVE PEROXIDES. EXTREMELY FLAMMABLE. Avoid breathing vapour and eye contact. O.E.L. 590 mg m-3.
Toxic effects The vapour irritates eyes and respiratory system; high concentrations have narcotic effect. Absorption or ingestion may cause liver damage.
Hazardous. Explosive peroxides formed on exposure to air/light. NaOH/KOH can cause explosion with peroxided material.
Fire hazardFlash point -17deg.C; ignition temp. 321deg.C; extinguish fire with CO2.
Spillage . Clear area, shut off all sources of ignition. Mop up with plenty of water and run to waste. Organise effective ventilation and evaporate remaining liquid.
DIETHYL ETHER: Colourless highly volatile liquid with characteristic odour; b.p. 34deg.C; immiscible with water. Liable to form explosive peroxides on exposure to light/air. Peroxides removed by treatment with aqueous sodium metabisulfite. HARMFUL VAPOUR. FORMS EXPLOSIVE PEROXIDES. EXTREMELY FLAMMABLE. Avoid breathing vapour. . O.E.L. 1200 mg m-3.
Toxic effects. Inhalation of the vapour may cause drowsiness, dizziness, mental confusion, faintness and, in high concentrations, unconsciousness. Ingestion may also produce these effects. Continued inhalation of low concentrations may cause loss of appetite, dizziness, fatigue and nausea.
Hazardous. Peroxide formation can result in subsequent explosion. Powerful oxidants can cause explosion. Reacts vigorously with sulfuryl chloride.
Fire Hazard. Flash point -45deg.C, ignition temp. 180deg.C; extinguish fire with CO2.
Spillage . Clear area, shut off all sources of ignition. Organise effective ventilation and allow to evaporate.
WARNING ON THE USE OF LITHIUM ALUMINIUM HYDRIDE (LAH)
1.All apparatus and reactants should be perfectly dry, and reactions should be run rigorously under nitrogen, with the reaction temperature below 70deg.C at all times.
2.Order of addition is important. Always first add the hydride to the solvent in the nitrogen purged apparatus before mixing with any reactant.
3.The hydride should never be allowed to form a crust above the level of the liquid or to settle to the bottom of a heated flask, so gentle efficient stirring is essential.
4.To prevent local overheating of the reaction vessel, heating mantles should never be used. Use an intermediate medium such as oil or graphite bath.
5.After reaction, destroy excess LAH by slow careful addition of ethyl acetate (preferably diluted with an inert solvent), under nitrogen, keeping the temperature below 70deg.C. All LAH reactions should be carried out behind protective screens.
Note that only one of these is to be carried out. The demonstrator will indicate which.
1. Reduction of Cinnamaldehyde to Hydrocinnamyl Alcohol: Normal Addition2
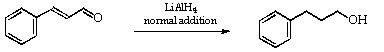
Dry, in an oven, a 500 ml three-necked flask, a 100 ml tap-funnel and a double surface condenser.
In a dry fume cupboard weigh out lithium aluminium hydride (5.8 g) and introduce it into a 3-necked flask via a powder funnel. Rinse in any residues with dry THF (~25 ml) and at once fit a stirrer, condenser and tap-funnel and set up the apparatus as in Figure 1. Use a Citenco brushless motor for the stirrer, a magnetic stirrer is not sufficiently powerful.
Pass a slow stream of purified nitrogen into the reaction flask, switch on the stirrer and heat the flask to a gentle reflux. From the dropping funnel add a solution of redistilled cinnamaldehyde (10 g) in dry THF (75 ml), very slowly and at such a rate that the THF in the flask maintains the gentle reflux. At intervals check the reaction mixture by tlc using silica plates developed in dichloromethane.
Figure 1
When the reaction is complete (> 10 min) cool the flask in water and ice and cautiously add saturated aqueous sodium sulfate (12 ml) dropwise to the stirred reaction mixture to decompose the excess of reagent. An aqueous solution is safe here because only a small quantity of reagent is present and it is in solution under an atmosphere of nitrogen. Then add 10% (v/v) sulfuric acid (95 ml). Separate the two layers (if present) and re-extract the aqueous layers with ether (4 x 30 ml). Dry the combined organic layers over anhydrous sodium sulfate.
Evaporate the solvent under reduced pressure (Rotavapor) and distil the residual oil under reduced pressure. Isolate the hydrocinnamyl alcohol (b.p. 120-121deg.C/13 mm Hg pressure). Record the yield, b.p. refractive index and i.r. and n.m.r. spectra and so confirm (or otherwise) the absence of cinnamaldehyde.
In the fume cupboard, clean any contaminated glassware by decomposing the lithium aluminium hydride with ethyl acetate.
2. Reduction of Cinnamaldehyde to Cinnamyl alcohol: Inverse Addition.3
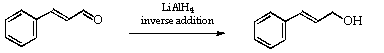
Dry, in an oven, a 250 ml three-necked flask, a 100 ml tap-funnel and a side arm adapter (see Figure 2).
Make up a solution/suspension of lithium aluminium hydride (0.72 g, 1.1 H- equiv) in dry ethyl ether (50 ml) in the 100 ml tap funnel. Set up the dried apparatus as in Figure 2 with a solution of cinnamaldehyde (redistilled, 10 g) in dry ether (25 ml) in the flask.
Figure 2
Switch on the stirrer, cool the flask with an ice-salt bath until the internal temperature is -10deg.C (pentane or alcohol thermometer) and add the solution/suspension of lithium aluminium hydride in ether during ~30 min so that the temperature remains below +10deg.C. Follow the reaction by tlc on silica plates developed in dichloromethane. If necessary add more lithium aluminium hydride in diethyl ether (carefully weighed out in the dry fume cupboard).
When the reaction is complete, add water (3 ml) to decompose the excess of the reagent and then add 10% (v/v) sulfuric acid (25 ml). Separate the ether layer and extract the aqueous layer with ether (2 x 50 ml). The combined layers are dried over anhydrous sodium sulfate. Evaporate the ether (Rotavapor) and by distil the residue under reduced pressure to isolate the cinnamyl alcohol (b.p. 139deg.C/14 mmHg). It should crystallise when cooled, m.p. 33deg.C.
Record the yield, refractive index, and nmr and infra-red spectra and interpret the latter. Transfer the material to a labelled tube.
If a sample fails to crystallise, purify it as follows: transfer the crude alcohol to a 250 ml conical flask, and dissolve it in dry ether (35 ml for each 5 g). Add powdered calcium chloride (10 g) and leave the mixture overnight under N2.
Next day collect the calcium chloride-cinnamyl alcohol solvate under N2, wash it with dry ether (20 ml) and then add it to water (25 ml). Extract the mixture with ether (3 x 25 ml) dry the extract over solid sulfate, filter the solution and evaporate the ether (Rotavapor) to obtain crystalline cinnamyl alcohol.
3. Disposal of Lithium Aluminium Hydride
Do this away from flames or other sources of ignition, best in the fume-cupboard, and WEAR GOGGLES OR A VISOR Dispose of the reagent, both solid and solution, by cautiously adding it to ethyl acetate under an atmosphere of nitrogen. NEVER add reagent residues to water or alcohols: there might be a violent explosion. Treat contaminated apparatus with ethyl acetate. Later, after at least 1 hour, wash the apparatus with water and dilute hydrochloric acid, and then rerinse it with water.
References
1. H.C. Brown and S. Krishnamurthy, Tetrahedron, 1979, 35, 567; E.R.H. Walker, Chem. Soc. Rev., 1976, 5, 23; N.G. Gaylord, "Complex Metal Hydrides", Wiley-Interscience, New York, 1956, p. 107.
2. R.F. Nystrom and W.G.Brown, J. Amer. Chem. Soc., 1947, 69, 1197, 2548; ibid., 1948, 78, 3738.
3. A.J. Hill and E.H. Nason, J. Amer. Chem. Soc., 1924, 46, 2236.