

AMMONIA(gas): Colourless gas with characteristic odour, b.p. -33deg.C. Miscible with water. TOXIC BY INHALATION. IRRITANT TO SKIN EYES AND RESPIRATORY SYSTEM. FLAMMABLE. Avoid breathing gas and skin contact. O.E.L. 17 mg m-1.
Toxic effectsThe gas irritates all parts of the respiratory system. Extremely irritating to eyes.
Hazardous. Reacts vigorously with oxidising agents. Mixtures with air can be explosive.
Fire hazardExplosive limits 16-25%; ignition temp. 651deg.C.
Disposal Clear area of people. Allow to evaporate in a well ventilated area, preferably a fume cupboard.
ACETYLENE: Colourless gas; b.p. -83deg.C; slightly soluble in water. EXTREMELY FLAMMABLE. EXPLOSIVE WITH OR WITHOUT AIR CONTACT. Avoid breathing gas. Asphyxiant, no O.E.L.
Toxic effects Primarily an asphyxiant.Inhalation of gas may cause giddiness, headaches and nausea. Narcotic when mixed with O2.
Hazardous Explodes alone or mixed with air. Reacts explosively with oxidising agents, halogens, potassium, HNO3 and Hg and Cu salts.
Fire hazard. Explosive limits 3-82%; ignition temp. 335deg.C; extinguish fire with CO2.
Disposal. Clear area of people. Allow to evaporate in a well ventilated area, preferably a fume cupboard.
SODIUM: Supplied as soft, silvery white sticks, normally coated with grey-white oxide/carbonate. Reacts vigorously with water generating H2 which may ignite.. DANGEROUS ON CONTACT WITH WATER. CAUSES BURNS. Avoid eye/skin contact.
Toxic effects. Contact with moist skin causes caustic and thermal burns.
Hazardous. Very reactive, explosively so with many acids. Dispersions in inert solvents. pyrophoric. Reacts violently with many organic halocompounds.
Spillage Clear area, shut off all sources of ignition. Wear face shield goggles and gloves. If contained, destroy cautiously with isopropanol. If scattered, cover with solid sodium carbonate, shovel into buckets and remove to safe place for destruction with isopropanol.
WARNING:--The formation of sodamide is exothermic and can cause the ammonia to boil vigorously. The reactions involving liquid ammonia must be performed in an efficient fume cupboard and gloves and goggles or a visor must be worn during these operations. It is important to use dry apparatus and to exclude moisture. Always use fresh rubber tubing to lead the ammonia from the cylinder to the reactor.
Liquid ammonia (b.p. -33deg.C) is a good solvent for many organic and inorganic substances, including the alkali and alkaline earth metals, and is therefore an excellent medium for reactions involving these. The liquid is slightly ionised:--

and the ammonium and amide ions are counterparts of the hydroxonium and hydroxide ions in water:--
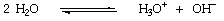
Therefore, in ammonia, ammonium salts and metal amides function as acids and bases respectively.
Organic reactions which can be carried out in liquid ammonia include: dehydrohalogenation with sodamide; debenzylation of O- and S-benzyl ethers and esters with sodium; reduction of alkynes to trans-alkenes with sodium and ethanol; metallations with sodium or sodamide. In particular, liquid ammonia is an excellent solvent medium for the formation and reactions of sodio-alkynes. The preparative usefulness of alkynes depends largely on this fact: acetylene (ethyne) itself gives, with sodamide in liquid ammonia, a monosodio- derivative whereas with a Grignard reagent it gives a dimagnesio- derivative (XMg-C[[equivalence]]C-MgX). Sodium acetylide and metallated alkynes (R-C[[equivalence]]C- M+; M = Na, Li, CaX) react, for example, with primary alkyl halides to give alkylalkynes and with aldehydes and ketones to form alkynylcarbinols.
Alkynylcarbinols derived from polyene aldehydes and ketones have been much used in vitamin A and carotenoid synthesis.
Preparation of Ethynylcarbinols from Acetylene.
The reaction stages are:--
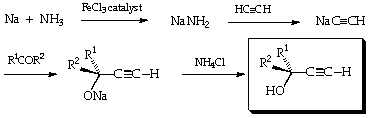
In consultation with a Demonstrator, choose one of cyclohexanone, acetone or benzaldehyde as your carbonyl reactant.
WARNING:-- The formation of sodamide is exothermic and can cause the ammonia to boil vigorously. The reactions involving liquid ammonia must be performed in an efficient fume cupboard and gloves and goggles or a visor must be worn during these operations. It is important to use dry apparatus and to exclude moisture. Always use fresh rubber tubing to lead the ammonia from the cylinder to the reactor.
a. Preparation of sodamide3
Consult Nick Davies before commencing this experiment. The condensation of the ammonia takes some time and must be started early in the morning. Nick will normally get this going for you. The apparatus must be set up the previous day and checked by Nick.
Set up the apparatus as shown in Figure 1 (a magnetic stirrer may be used as an alternative to the mechanical one shown) in the designated fume cupboard, making sure that the stand and clamps are secure.
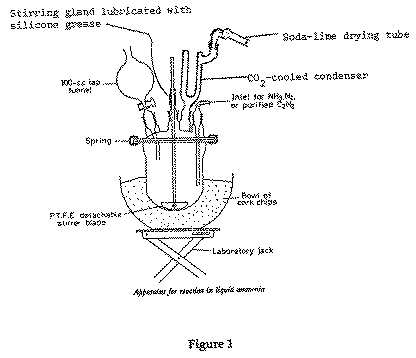
For thermal insulation of the 700 ml flanged reaction vessel (a four-necked lid is required, see Figure 1) use a bowl of cork chips mounted on a laboratory jack. Fill the outlet drying tube on the top of the 'cardice' condenser with sodalime (14-20 mesh granules), do not use calcium chloride because this reacts with ammonia. Connect the inlet tube to the ammonia cylinder via a sodalime drying tube.
{The following part will normally be carried out by Nick Davies. Fill the 'cardice' condenser with solid CO2 pellets and then carefully open the ammonia cylinder. Liquefied ammonia will be produced by the 'cardice' condenser. In this manner distil ~500 ml of ammonia into the reactor (premark a 500ml level on the side of the vessel). The condenser will need topping up with solid CO2 pellets from time to time}
Cut clean sodium (5.74 g) into small pieces under xylene in a flat petri dish. Connect the inlet to a nitrogen supply and pass a slow stream of N2 over the ammonia. Start the stirrer and add finely powdered hydrated ferric nitrate (0.1 g) and then sodium (0.1 g). After a varying amount of induction time, the colour of the liquid changes from dark blue to grey/black as the catalytic Fe metal is generated and hydrogen is evolved. As soon as this happens, start adding the small pieces of sodium metal slowly but steadily. (Do not delay this addition or the sodium may not continue to react. In this event, more iron salt may be added but the solution will become very dark. Equally, do not pile in too much sodium or the mixture may boil up vigorously as the iron catalyst takes effect).
Adding the remaining sodium during 15-20 min and continue stirring until the solution reverts to grey/black (25-100 min) when formation of the sodamide is complete. Keep the condenser well topped up with 'cardice' during this period.
b. Preparation of sodium acetylide:-- Connect the acetylene cylinder to the reactor inlet tube via a mercury safety valve, a trap cooled with a 'cardice'/alcohol bath to condense out acetone (acetylene is stored in the
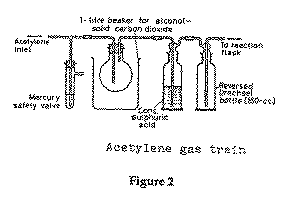
cylinder as a compressed solution in acetone to prevent explosive polymerisation; the acetone could be an unfortunate impurity in this experiment if it is not carefully removed), a conc. sulfuric acid trap and a reversed Drechsel bottle (see Figure 2). A flowmeter can be included between the last Drechsel bottle and the reactor.
Pass a rapid stream of acetylene (~1 l/min) until the colour of the reaction mixture darkens(~1 h). The formation of sodium acetylide is then complete. Condense more ammonia into the reactor if the volume has fallen below ~250 ml.
c. Reaction of the sodium acetylide with a carbonyl compound:-- Reduce (or recommence) the flow of acetylene to ~ 0.5 l/min). Add dropwise a solution of the purified4 carbonyl compound (cyclohexanone 25g; acetone 15 g; or benzaldehyde 26 g) in dry ether (25 ml) via the tap funnel and continue stirring for a further 3 h.
When the reaction is complete, close the valve on the acetylene cylinder, replace the gas inlet tube on the reactor with a stopper and add ammonium chloride (15 g) in small portions over 15 min. CARE the reaction is strongly exothermic!
If necessary, the reaction can be left overnight at this stage provided that the reactor is topped up with ether and the stirrer is switched off. Do not allow the ammonia to evaporate completely as the crude alkynylcarbinol would polymerise.
Remove the condenser, switch on the stirrer and replace the cork chip bath with a warm water bath to evaporate the ammonia rapidly but without splashing. Without delay, add ether (50 ml), [if ether has been added the evening before, simply reduce the volume to ~ 100ml] filter the mixture and thoroughly wash the solid on the filter with ether (150 ml).
Distil off ~100 ml of ether from the combined filtrate and washings, to expel any ammonia remaining, and shake the residual ether solution with saturated aqueous sodium hydrogen sulfite, prepared from sodium metabisulfite (24 g) and water (40 ml). filter the mixture and wash the solid on the filter with ether. Dry the ethereal solution, first over anhydrous sodium sulfate and then over anhydrous potassium carbonate, and filter off the drying agents. Remove the solvent (Rotavapor), transfer the residue to a distillation flask containing potassium carbonate (~0.1 g) and fractionally distil the product under nitrogen, if necessary under reduced pressure (see below). Note that, above 100deg.C, potassium carbonate may cause disproportionation of an acetylenic carbinol but that below this temperature its use avoids the danger of acid catalysed polymerisation of the product.
d. Purification of the product
i. Cyclohexanone product:-- Redistil and collect the fraction b.p. 76-9deg.C / 10 mm of Hg. Record the yield, i.r. and n.m.r. spectra.
ii. Acetone product:-- Redistil and collect the fraction b.p. 102-104deg.C / 760 mm of Hg. If the product has not been sufficiently dried, the azeotrope with water (25% carbinol) is obtained, b.p. 90deg.C / 760 mm of Hg. The water can be removed by further drying with anhydrous potassium carbonate. Record the yield, i.r. and n.m.r. spectra.
iii. Benzaldehyde product:-- Redistil from potassium carbonate (0.1 g), b.p. 94deg.C / 0.7 mm of Hg. The product crystallises on cooling so be prepared to warm the condenser (hair dryer) if it threatens to become blocked with solid. The carbinol may be recrystallised from ether/petroleum ether (b.p. 40-60deg.C) at -40deg.C. Collect the solid on a precooled filter. Record the yield, i.r. and n.m.r. spectra.
References
1. G.W. Watt, J. Chem. Ed., 1957, 34, 533.
2. H. Smith, 'Organic Reactions in Liquid Ammonia', Academic Press, New York, 1963.
3. K.W. Greenlee and A.L. Henne, Inorg. Synth., 1940, 2, 128.
4. D.D. Perrin, W.L.F. Armarego and D.R. Perrin, 'Purification of Laboratory Chemicals', 2nd edn, Pergamon Press, Oxford, 1980.