A4: CATALYTIC HYDROGENATION AT ATMOSPHERIC PRESSURE.
Hazard Data
HYDROGEN: Accidental leakage of H2 gas may cause an explosion. Do
not bring any ignition sources into the vicinity of the apparatus and always
vent the gas burette outside the window. Ensure that a key is fitted to the
cylinder regulator at all times to minimise escape of H2 gas in the
event of a rupture of the regulator diaphragm. Turn the hydrogen off at the
regulator whenever the apparatus is not in use. Ensure that the cylinder is
properly clamped.
PLATINUM Platinum metals are very toxic and platinum
compounds
METALS:themselves are known mutagens. Avoid ingestion,
inhalation of the dust and skin contact. Wash hands thoroughly with soap and
water if contamination occurs. Spillage of small quantities which are not
recoverable should be washed to waste with copious quantities of water.
AT NO STAGE SHOULD A 'USED' CATALYST BE ALLOWED TO DRY OUT. THE METAL
WILL BE SATURATED WITH HYDROGEN AND CONTACT WITH AIR MAY CAUSE A FIRE OR
EXPLOSION.
Detailed information: BDH Hazard Data Sheets, p. 828
(Pt) and p. 758 (Pd).
MERCURY:Mercury is a toxic metal. Some of the equipment
contains liquid mercury. In the event of a spillage, inform the Technician or a
Demonstrator who will supervise its removal.
Detailed information: BDH Hazard Data Sheets, p.
642.
QUINOLINE:Quinoline is toxic. Avoid ingestion, inhalation and
skin contact. Wash thoroughly with water if contact occurs. O.EL. not given
Detailed information: BDH Hazard Data Sheets, p.
895.
Introduction1
The direct addition of hydrogen to olefinic and acetylenic linkages, the
reduction, with hydrogen, of some unsaturated functional groups (e.g. -NO2
-> -NH2; -CN -> -CH2NH2; -CH=NOH -> -CH2NH2) and the hydrogenolysis of
others (e.g. -N=N- -> 2 -NH2; ROCH2Ph -> ROH + MePh) can be effected at
ordinary temperature and pressure with the aid of either heterogeneous or
homogeneous catalysts. For example, a solution of the compound in water,
ethanol, methyl or ethyl acetate or acetic acid may be shaken with hydrogen gas
either in the presence of platinum, palladium, rhodium or Raney nickel, as the
heterogeneous catalyst. Adam's platinum catalyst, with acetic acid as solvent
for the substrate, is a powerful combination with which even aromatic rings may
be hydrogenated at ordinary temperature and pressure. Supported catalysts and
partially poisoned catalysts (e.g. Lindlar's palladium catalyst) are more
selective. They enable partial reduction (such as -C[[equivalence]]C- ->
-CH=CH-), or hydrogenolysis without reduction of unsaturated groups to be
accomplished.
More recently, organometallic complexes such as
chloro(tristriphenylphosphine)rhodium(I) (Wilkinson's catalyst) have found wide
application as homogeneous catalysts. Often these are more efficient than their
heterogeneous counterparts.2
Catalysts
Prepare one of the following catalysts, as appropriate to your
chosen hydrogenation reaction, following consultation with a Demonstrator:--
Adam's Catalyst3
In a fume cupboard, dissolve chloroplatinic acid (H2PtCl6.6H2O,
0.10 g) in water (ca. 0.5 ml) in a porcelain crucible (3-4 cm diameter).
Add sodium nitrate (1 g) and evaporate the mixture to dryness, over a low
flame, with continuous stirring. Turn the Bunsen burner full on and stir the
contents of the crucible vigorously until the mass has melted completely and
the initial decomposition has subsided. Keep the bottom of the crucible at a
dull red heat for a further 30 min. (too strong a heat decomposes the oxide to
the metal). Allow the crucible to cool and wash the contents into a 250 ml.
beaker with hot water from a wash bottle. Filter off the brown platinum oxide
with a small ('Hirsch') funnel (Whatman paper No. 541) and wash the oxide with
hot water (about 200 ml) until the washings are free from nitrate ion. Dry the
catalyst over calcium chloride in a vacuum desiccator.
Palladium Black4
Heat a mixture of palladium chloride (0.50 g) and water (100 ml) to
80deg.C and carefully neutralise the suspension, to wide-range indicator
paper, with 20% sodium hydroxide solution (if the end point is overshot,
palladium hydroxide precipitates). Add 2.6% formic acid (2.5 ml) and, after
about 2 min. make the solution strongly alkaline with 20% sodium hydroxide
solution (5 ml). Add more of the formic acid (5 ml) and make sure that the
solution is still alkaline. Heat the mixture on a steam bath for 2 hours.
Filter off the precipitate with a small ('Hirsch') funnel (Whatman filter paper
No. 541), wash it free from alkali and dry it over calcium chloride in a vacuum
desiccator.
5% Palladised Charcoal5
Heat decolourising charcoal (7.5 g) on a steam bath for 2-3 hours with conc.
hydrochloric acid (5 ml) and water (150 ml). Wash the charcoal by decantation
with hot water until free of acid, filter it off, and dry it in an oven at
<100deg.C.
Warm palladium chloride (0.5 g) in conc. hydrochloric acid (0.75 ml) and water
(5 ml) on the steam bath for ~ 20 min. Add the solution to AnalaR sodium
acetate (17.5 g) in water (50 ml) contained in a hydrogenation flask. Introduce
the purified charcoal (5.8 g) and hydrogenate the mixture until no more
hydrogen is absorbed (~ 2 h). The hydrogenation procedure is described below.
Collect the catalyst on a 7 cm Buchner filter (3 thicknesses of Whatman No. 1
filter paper), wash it with water (5 x 100 ml) and drain it on the filter with
suction. Dry the catalyst over fresh silica gel in a vacuum desiccator and
store it in a tight stoppered bottle.
Lindlar's Catalyst (5% Pd on CaCO3, poisoned with Pb)6
Stir calcium carbonate (precipitated, light; 1.1 g) in water (10 ml) in a
flask which is fitted with a thermometer and mounted on a magnetic
stirrer/hotplate. Whilst continuing the stirring, add palladium chloride (90
mg) to the suspension and after 5 min., raise the temperature to 80deg.C for a
further 10 min. Cool the mixture and transfer it to a hydrogenation flask,
using ~ 10 ml of water for rinsing. Hydrogenate the mixture until hydrogen
absorption (10-20 ml) is complete (ca. 15 min); the hydrogenation
procedure is described below. Collect the reduced catalyst on a small 'Hirsch'
funnel (Whatman filter paper No. 1) and wash the catalyst with distilled water
(20 ml). Add the damp catalyst to distilled water (10 ml) in a flask on the
magnetic stirrer/hotplate. Switch on the stirrer, add a 5% (w/v) solution of
AnalaR lead acetate in water (2 ml) and, after 10 min., raise the temperature
to 90deg.C for 40 min. During this time, add distilled water, as necessary, to
compensate for evaporation losses. Cool the mixture, collect the catalyst on
Whatman No. 1 paper in a small 'Hirsch' funnel and wash the catalyst with
distilled water (total, ~ 50 ml). Transfer the catalyst to a clean, tared,
specimen tube and dry the catalyst in a pistol at 40deg.C to constant weight (~
1-3 h). Crush the dry catalyst to a powder with a clean spatula or glass rod
and stopper and label the tube (catalyst yield: 0.9-1 g).
Hydrogenation
Complete either 1, 2 or 3 after consultation with a Demonstrator.
1. Hydrogenation of an Olefinic Bond: Use of either Pd Black or Pd on
Charcoal.
Discuss your choice of catalyst with a Demonstrator. The experiment
will be carried out on the macro-scale atmospheric pressure hydrogenator.
HO2CCH=CHCO2H + H2 -> HO2CCH2CH2CO2H
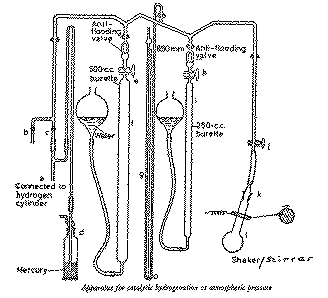
 Preliminaries.-- Examine the taps and ensure that they are well
fitting, properly greased and that the bores are free of obstruction. Apply, as
necessary, a thin smear of grease around the upper part of the cone
k (do not attach the hydrogenation flask yet). See that
there is sufficient water (containing a small amount of copper sulfate to
suppress algal growth) in the reservoirs attached to the burettes
f and i. Connect tap
b to the water pump, or other source of vacuum, via
a trap and a 3-way stopcock. Connect the hydrogen cylinder regulator outlet to
the the inlet a with pressure tubing. There is a mercury
safety bubbler at d which prevents overpressurisation of
the apparatus.
Preliminaries.-- Examine the taps and ensure that they are well
fitting, properly greased and that the bores are free of obstruction. Apply, as
necessary, a thin smear of grease around the upper part of the cone
k (do not attach the hydrogenation flask yet). See that
there is sufficient water (containing a small amount of copper sulfate to
suppress algal growth) in the reservoirs attached to the burettes
f and i. Connect tap
b to the water pump, or other source of vacuum, via
a trap and a 3-way stopcock. Connect the hydrogen cylinder regulator outlet to
the the inlet a with pressure tubing. There is a mercury
safety bubbler at d which prevents overpressurisation of
the apparatus.
Procedure.-- Dissolve maleic acid (3 g) in ethanol (40 ml) in a
hydrogenation flask (250 ml) and add the catalyst palladium black (30 mg)
or palladised charcoal (100 mg) and a magnetic stirrer bar. Calculate
the expected uptake of hydrogen in order to determine whether the gas burettes
will require refilling during the experiment.
Open tap j. Raise the reservoirs attached to the burettes
and thus fill the burettes with water up to the taps e and
h. Close taps e and
h. Lower the reservoirs.
Attach the charged hydrogenation flask l at
k and fit the safety spring across the joint and place a
magnetic stirrer under the flask.
With the tap a open, and taps c,
e and h closed, evacuate the apparatus
with the water pump vacuum via tap b.
Close tap b and fill the apparatus with hydrogen,
via tap c, to atmospheric pressure (indicated on
manometer g). Close tap c.
Re-evacuate the apparatus through tap b and then close
b.
Refill with hydrogen through tap c, then carefully open
taps e and h so that the burettes will
fill with hydrogen. Close tap c.
With taps b and c closed and
e, h and j open,
level the water in the reservoirs against that in the burettes (to bring the
hydrogen to atmospheric pressure). Note the water levels in the burettes (total
volume, R0). Close the tap h.
Switch on the stirrer and from time to time, adjust the level of the reservoir
attached to the burette f so as to maintain the hydrogen at
a pressure slightly greater than atmospheric. At intervals, level the water in
the reservoir against that in the burette(s), stop the stirrer and note the
reading(s) (total volume, Rt). Restart the stirrer.
Plot the hydrogen uptake Rt (in ml) against time (in min).
When the hydrogen is almost used up, level off the reservoir, take the burette
reading, and close tap e. Open tap h
and use the hydrogen in i.
When absorption of hydrogen ceases, adjust the level of the reservoir of
i and read the burette. Close tap h and
switch off the stirrer.
With taps e and h closed, evacuate the
apparatus through tap b. Admit air through
b and remove the flask l.
Filter off the catalyst through a thin layer of acid-washed Kieselguhr filter
aid in a Hirsch funnel, wash it with a little ethanol and place the catalyst,
together with the filter paper and filter aid in the catalyst residue bottle.
Evaporate the filtrate under reduced pressure, recrystallise the residue
(water) and determine the yield, m.p., i.r. and n.m.r. of the pure product.
Interpret these data.
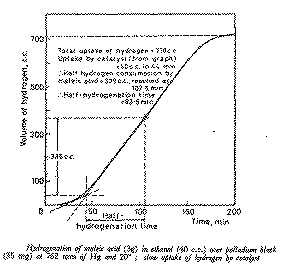
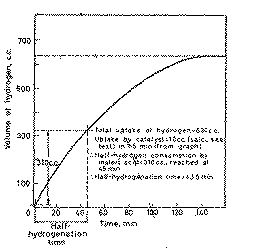
From the hydrogenation curve, determine the activity of the catalyst,
(expressed as the half-hydrogenation time, tH1/2, see Figure 2) and the total
hydrogen uptake by the substrate and the catalyst as demonstrated in Figure 2.
Submit the excess catalyst, labelled with its identity and activity (tH1/2),
along with the product, for assessment.

2. Semi-hydrogenation of an Acetylenic Bond: Use of Lindlar's Catalyst.
Lindlar's catalyst, additionally poisoned with quinoline, is used to
semi-hydrogenate 3-phenylpropyn-3-ol using the macro-scale atmospheric
hydrogenator.
PhCH(OH)C[[equivalence]]CH + H2 -> PhCH(OH)CH=CH2
Preliminaries.-- See above
Procedure.-- Dissolve 3-phenylpropyn-3-ol (5 g) in toluene (50 ml) in a
hydrogenation flask (250 ml) and add Lindlar's catalyst (150 mg) and quinoline
(400 mg).
Calculate the theoretical uptake of hydrogen at room temperature and pressure
in order to determine whether the gas burettes will require refilling during
the experiment.
Hydrogenate the substrate as described in Experiment 1 above until ~1.05
equivalents of hydrogen have been absorbed.
Plot the uptake of hydrogen (in ml) against time (in min) and note the abrupt
change of rate when semihydrogenation is complete.
Filter off the catalyst with a Hirsch funnel (Whatman No. 1 paper), wash it
with toluene (~5 ml) and place the used catalyst in the correct residue bottle.
Evaporate the filtrate under reduced pressure (Rotavapor) and distil the
residue with a Büchi kugelrohr apparatus.
Record the b.p., yield and i.r. and n.m.r. spectra and interpret the latter.
3. Hydrogenation of Cholesterol: Use of Adam's Catalyst.
The sterol is hydrogenated with Adam's catalyst in the
micro-hydrogenation apparatus.
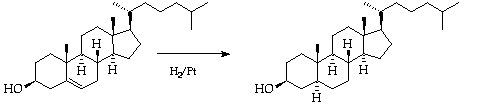
Preliminaries:--Purify a sample (~ 500 mg) of cholesterol by
recrystallisation from ethanol and drying it in a vacuum pistol overnight.
Clean, as necessary, and lightly grease the joints J1 and J2 with
Apiezon M grease.
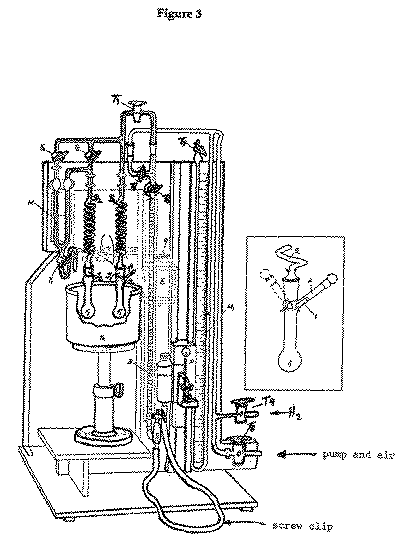
Procedure:--Ensure that the tap T1 is closed and that the
indicator liquid levels in the manometer M are near the middle of the
scale. Place THF (10 ml), acetic acid (1 drop) and purified cholesterol
(<=80 mg, weighed accurately) in flask F1 and a similar volume of
THF/AcOH only in flask F2. Calculate the expected uptake of hydrogen
from the weight of cholesterol taken. (Correction to STP has to be made in
estimating the volume, the accuracy of the burette is 4%). This volume should
be less than 5.5 ml, the capacity of the burette. Connect them to the apparatus
using the springs provided.
Weigh out ~5 mg of Adam's catalyst in one of the small tubes provided. Ensure
that the flask F1 is so rotated with respect to the joint J1 that
the side arm A is closed off from the flask and then carefully place the
tube containing the sample in the side arm. Replace the side arm stopper.
With the taps T2, T3, T4, T5, T6 and
T7 open and T9 closed, and with the mercury kept at the bottom of
the reservoir B by means of the closed screw clip on the connecting
rubber tubing, slowly evacuate the apparatus through tap T8, which is
connected to a vacuum (water) pump. Agitate the liquid in flasks F1 and
F2 by tapping them occasionally whilst they are being evacuated, in
order to remove dissolved or occluded air, but avoid evaporating too much
solvent. Close the tap T8.
Carefully turn on the stream of hydrogen until it bubbles through the mercury
in the safety bubbler (not illustrated); a fast but steady stream of gas is
required. After a minute or two, slowly open the tap T9 so that the
hydrogen enters the system until the whole apparatus, including the burette, is
filled with gas to atmospheric pressure (indicated by the manometer).This
operation must be performed carefully in order to avoid suck-back of the
mercury in the bubbler. Close the tap T9 to the hydrogen supply.
The sequence of evacuation and hydrogen admittance must be repeated at
least 2 more times in order to effect complete deoxygenation of the gas
in the apparatus. However, in the subsequent purging operations, prolonged
evacuation of the system is not necessary.
Release the screw clip om the burette rubber tubing and adjust the level of
the mercury reservoir R until the mercury just comes to the calibrated
part of the burette B. Close taps T3 and T5 and turn off
the hydrogen supply at the cylinder head. Commence shaking the flask and after
2-3 min. check that the burette reading remains constant (no leaks/uncatalysed
uptake by substrate).
Stop the shaker, adjust, as necessary, the reservoir R height until the
level of the liquid in the 2 arms of manometer M is equal and note the
burette reading (R0). Rotate the flask F1 on its joint until the tube
containing the catalyst drops into the flask. Carry out this operation as
smoothly and quickly as possible and avoid holding the flask or glass spiral
more than absolutely necessary to minimise heating by the hand. A slight drop
in the level of the right hand limb of the manometer M can be ignored.
Recommence the shaking of the flask. As reduction proceeds, the level in the
left hand limb of manometer M rises. At regular time intervals,
(initially 2 min, later 5 or 10 min) readjust the mercury level in R to
rebalance M and take a burette reading. Record the time and the burette
reading (Rt) and plot a hydrogenation curve (see 1 above). Continue until
hydrogen uptake (by catalyst + substrate) ceases.
Quickly lower the mercury reservoir R and reclose the screw clip. Open
T3 and T5 and evacuate the apparatus via tap T8
before releasing air into the system. Remove the flasks F1 and
F2.
N.B. During reduction and when the system is not being handled, ensure that
the cupboard doors are closed. This protects the apparatus from draughts
and therefore from misleading fluctuations in the internal pressure.
Filter the solution from the flask F1 through a plug of neutral alumina
and wash the product through with diethyl ether. Evaporate the combined
filtrates on a Rotavapor at <=50deg.C and recrystallise the product from
aqueous ethanol. Dry in a drying pistol overnight at ~50deg.C.
Record the m.p., specific rotation (as [[[alpha]]]D = 100[[alpha]]/cl; c in
g/100ml; l= pathlength in decimeters), and nmr and i.r. spectra of the product
and compare with those of the starting substrate.
From the hydrogen uptake/time plot deduce the volume absorbed by the catalyst
and the half-hydrogenation time (see Figure 2).
References
1. There are many excellent reviews on catalytic hydrogenation. For a
recent example, see Jerry March, 'Advanced Organic Chemistry', 4th edn, John
Wiley and Sons, 1992, p 771 and references cited there.
2. For a comprehensive and well referenced review, see P.A. Chaloner, 'Handbook
of Coordination Catalysis in Organic Chemistry', Butterworths, London, 1986, pp
9 - 217.
3. R. Adams, V. Voorhees and R.L. Shriner, Org. Synth., 1941, 1,
463.
4. H. Wieland, Chem. Ber., 1912, 45, 484.
5. B.S. Furniss, A.J. Hannaford, P.W.G. Smith and A.R. Tatchell in 'Vogel's
Textbook of Practical Organic Chemistry', 5th edn, Longman, London, 1989, p.
452.
6. A. Lindlar, Helv. Chim. Acta, 1952, 35, 446; see also
R.L. Augustine in 'Catalytic Hydrogenation: Techniques and Applications in
Organic Synthesis'
Copyright (c) H. S. Rzepa, S. Marsden and ICSTM Chemistry Department, 1994-7.
 Preliminaries.-- Examine the taps and ensure that they are well
fitting, properly greased and that the bores are free of obstruction. Apply, as
necessary, a thin smear of grease around the upper part of the cone
k (do not attach the hydrogenation flask yet). See that
there is sufficient water (containing a small amount of copper sulfate to
suppress algal growth) in the reservoirs attached to the burettes
f and i. Connect tap
b to the water pump, or other source of vacuum, via
a trap and a 3-way stopcock. Connect the hydrogen cylinder regulator outlet to
the the inlet a with pressure tubing. There is a mercury
safety bubbler at d which prevents overpressurisation of
the apparatus.
Preliminaries.-- Examine the taps and ensure that they are well
fitting, properly greased and that the bores are free of obstruction. Apply, as
necessary, a thin smear of grease around the upper part of the cone
k (do not attach the hydrogenation flask yet). See that
there is sufficient water (containing a small amount of copper sulfate to
suppress algal growth) in the reservoirs attached to the burettes
f and i. Connect tap
b to the water pump, or other source of vacuum, via
a trap and a 3-way stopcock. Connect the hydrogen cylinder regulator outlet to
the the inlet a with pressure tubing. There is a mercury
safety bubbler at d which prevents overpressurisation of
the apparatus.




