| |
 
Background
It is possible to reproduce the active region of a naturally occurring
protease inhibitor protein, Bowman Birk Inhibitor, by synthesis of cyclic
peptides that encompass the active site of this protein. These peptides retain
the structural features of the original protein and most of the inhibitory
activity, but are easy to synthesize by automated solid phase methods.
Strategy
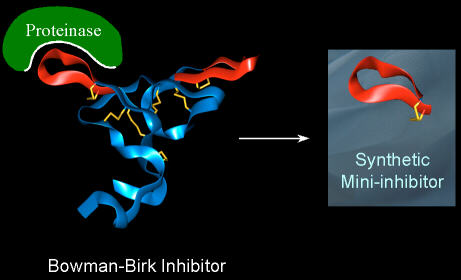
The backbone of the Bowman-Birk Inhibitor protein is shown as a ribbon. The
molecular structure has a pseudo-two fold symmetry, and two active sites of very
similar structure are present per molecule (shown in red). The interaction with
the protease involves the formation of a non-covalent complex, shown
schematically in the figure. The inhibitor contains many disulphide bridges
(yellow), and the active site loop is naturally delimited by one of these
disulphides. Synthesis of the loop in isolation (right) generates a short
peptide, typically 9-11 residues long, that retains the structural features of
this region from the parent protein, and demonstrates inhibitory activity.
|