Introduction
Photosystem II (PSII) has the unique ability to create a high enough oxidising potential that allows the oxidation of water to oxygen. We are interested in studying both the primary processes and the water splitting reactions that take place in Photosystem II. Photosystem II comprises over twenty identifiable polypeptides, most of which are membrane proteins. At the heart of PSII lies the reaction centre (see figure 1). The reaction centre undertakes the first energy conversion step of photosynthesis by using absorbed photons to drive a series of energy and electron transfer reactions, which ultimately result in a separation of charge. These reactions occur over timescales that cover more than fifteen orders of magnitude. This charge separation drives both water splitting and the creation of a transmembrane potential difference across the thylakoid membrane.
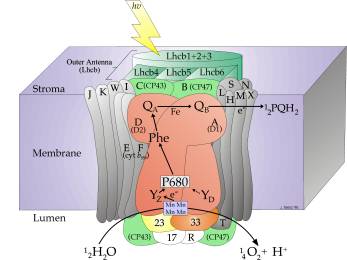
Figure 1.

Primary Dynamics in Photosystem II
Energy transfer and light induced charge separation are the primary photochemical events of photosynthesis. Our studies on isolated reaction centre samples, have allowed these primary reactions to be investigated without the added spectral congestion caused by the presence of antenna or secondary electron transfer pathways. Figure 2 qualitatively summarises the energy equilibration and primary electron transfer processes that take place in the Photosystem II reaction centre.
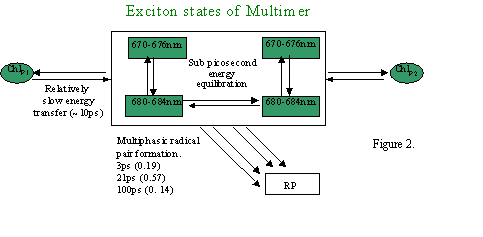
Figure 2.
Efficient charge separation in photosynthetic reaction centres requires the balancing of these electron and excitation energy transfer processes, which in Photosystem II are particularly closely entangled. Calculations, which treat the cofactors of the Photosystem II reaction centre as a supermolecular complex, allow energy and electron transfer reactions to be described in a unified way. We have shown this calculational approach to be in good agreement with experimentally observed energy and electron transfer dynamics. Indeed, as figure 3a and b demonstrate, this supermolecular view can correctly predict the effect of changing the redox potentials of cofactors by site directed mutagenesis. This work has shown that we have a unified and quantitative understanding of the structure-function relationship of the PSII reaction centre.
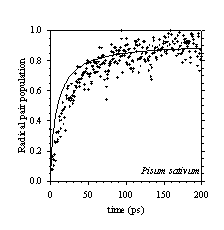 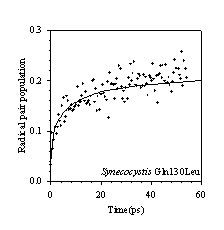
Figure 3: Comparison of experimental kinetics of radical pair formation obtained using transient absorption spectroscopy, with Multimer Model calculations for (a) PS II wild type reaction centres isolated from Pisum sativum, (b) D1-Gln130Leu genetically engineered reaction centres isolated from the cyanobacterium Synechocystis 6803.
Concerns have been raised regarding how far the photochemical properties of the remaining pigments in isolated reaction centre complexes have been altered in comparison to intact Photosystem II. We have carried out work to investigate this, and have been able to demonstrate that the trapping action of the primary radical pair formation in isolated Photosystem II reaction centres which contain eight chlorophyll molecules, is indeed identical to that found in larger Photosystem II particles with QA in a closed state, even up to membrane fragments (BBY’s) which contain ~250 molecules of Chlorophyll.
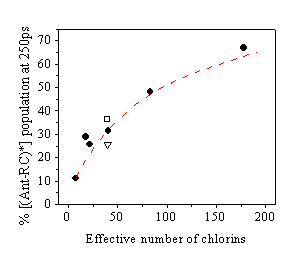
Figure 4: Singlet state populations obtained from fits to time resolved single photon counting data obtained from higher plant samples (·) are compared with the model results obtained using the three-state phenomenological kinetic model that allows inhomogeneous broadening of the radical pair free energies (----). Data from Synechocystis 6803 wild type (□) and D1-His198Ala (∇) dimeric CP43/CP47 core complexes are also shown for comparison.

Water splitting in Photosystem II
Water splitting occurs via a period four reaction, which causes oscillations in the kinetics as a function of the excitation flash number. The water splitting reactions include the re‑reduction of a chlorophyll species, P680, by a tyrosine residue. The free energy of this radical pair state relaxes via the movement of a proton within the protein. Figure 4 below shows that the oscillations on the nanosecond timescale are not effected by the deuteration of the protein but the microsecond oscillations shown in figure 5 are clearly affected, indicating that the microsecond phases are rate limited by proton/hydrogen transfer reactions.
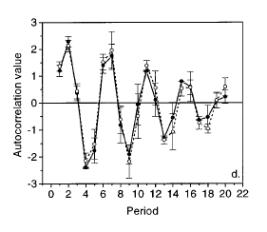
Figure 5.
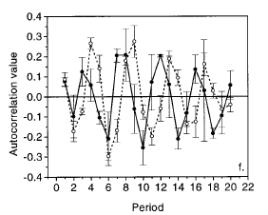
Figure 6.
The electron transfer process between the P680 and the tyrosine residue in Photosystem II is illustrated in Figure 7, showing an initially unstable state after electron transfer which is stabilised after proton rearrangements in the protein.
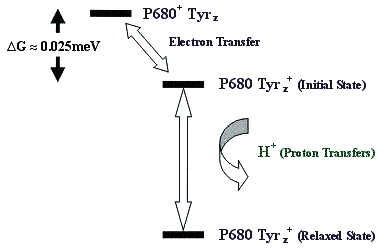
Figure 7.

|
