Introduction
To address the issues of fossil fuel depletion and the environmental problems caused by their combustion, and also to support the sustainable development of mankind, a clean and renewable energy is urgently required. To this end, hydrogen is a favourable energy source for helping to meet the world’s future energy demands. Compared with hydrogen production from fossil fuels or natural gas, light-driven hydrogen production is much cleaner and so much research has been aimed at photocatalytic water splitting. At present the quantum efficiency of this process is still quite low (QE <1%) and so is considerably below what is required for its practical application. A better understanding of the mechanism of water splitting is required if the QE of this process is to be improved. To date a clear picture of the photocatalytic hydrogen and oxygen production from water has not been developed. The recombination of photogenerated charge carriers is probably a key issue in restraining the QE, but no unambiguous correlation has been observed. In particular, oxygen production is assumed to require four holes simultaneously (a more complicated mechanism compared to that of hydrogen production), though this has not yet been confirmed. Our work therefore focuses on discovering the details of the mechanism of photocatalytic water splitting and subsequently trying to improve the QE of photocatalysts for solar hydrogen production.

Instruments
Pulsed- and frequency-controlled femto, pico and nano-second laser systems. Photocatalytic water splitting system connected with online real time analysis setup.
Schematic Diagram:
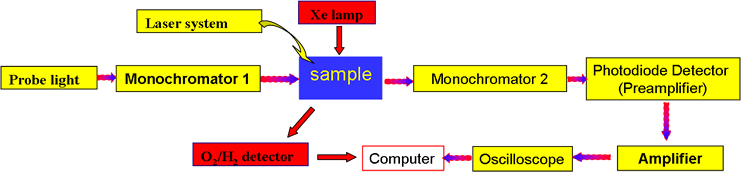

Experimental setup with Xe Lamp
 
Detail of the reaction cell on the O2/H2 detector (left) and the TiO2 film inside the cell (right)

Results
Nanocrystalline TiO2 film
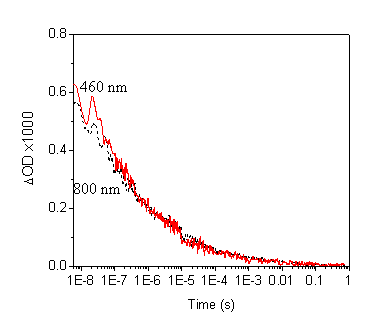
Fig. 1 Transient absorption spectra of nanocrystalline TiO2 film after laser excitation of 0.35 mJ/cm2 in argon atmosphere.
The figure 1 indicates the natural recombination of photoelectrons and photoholes without other quenching in the as-prepared TiO2 film.
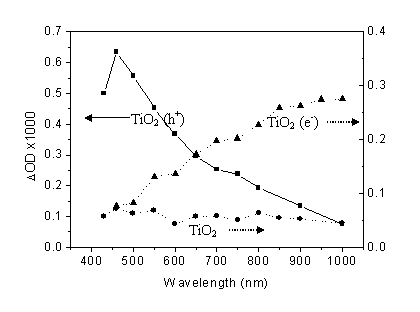
Fig. 2 Wavelength dependence of transient absorption of laser-excited charge carriers in nc-TiO2 film. Date was collected at 20µs for photogenerated electrons (TiO2 (e-)) with methanol as hole scavenger and holes (h+) with Pt as electron scavenger in argon.
Holes and electrons both have broad absorption (figure 2). The hole absorption peaks at 460nm and the electron absorption increases with increasing wavelength.

|
