Probing the active site of bacteriorhodopsin using electronically enhanced DOVE-FWM
Resonance Electronic Enhancements of DOVE-FWM
It is possible to achieve enhancements of DOVE-FWM signal if the sample has a suitable electronic resonance that can also be probed.
DOVE-FWM signal is generated when the two infrared beams, Eα and Eβ, are resonant with coupled vibrational transitions of the sample. In addition, it is possible for the visible beam, Eγ, to be in resonance with an electronic state. If this state is coupled to the vibrational modes being probed by the infrared beams, then the DOVE signal will be enhanced. This DOubly Vibrationally Enhanced Singly Electronically Enhanced Four Wave Mixing (DOVESEE-FWM) is a triple resonance technique and the resulting spectra are 3D maps of vibration-vibration-electron couplings.
The DOVESEE-FWM technique has the potential for homing-in on the active sites of proteins if these contain a group with appropriate electronic transitions, for example metal ions or chromophores. Due to the triple enhancement of the FWM signal that could be achieved from such groups, it would be possible to selectively study these whilst avoiding the surrounding protein. Although such groups represent only a tiny part of the protein, their signals could be picked out above those from the rest of the large protein.

Bacteriorhodopsin
Bacteriorhodopsin (bR) is a small (26kDa) transmembrane protein located in Halobacterium salinarium’s photosynthetic purple membrane (PM). It acts as a photon-driven proton pump by converting the energy of green light into an electrochemical proton gradient, which is then used by a second protein, ATP synthase, to generate chemical energy in the form of ATP. Each 248 residue-long bR chain folds into seven transmembrane alpha helices with short, extra-membrane interconnecting loops. At the heart of the protein lies its photoactive moiety, an all-trans retinal molecule, which is covalently bound to the ε-amino group of a conserved lysine (Lys-216) via a protonated Schiff base. Upon light excitation, bR progresses through a photocycle comprised of a number of structurally distinct intermediates in which the retinal chromophore adopts various conformations.
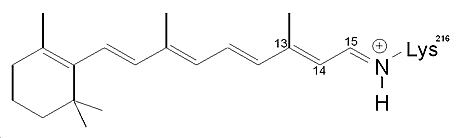
bR's retinal chromophore

Probing the bR Retinal Using Electronically Enhanced DOVE-FWM
Via a resonance electronic enhancement of its DOVE-FWM signal it is possible to selectively probe bR’s retinal molecule. The DOVE 2D-IR spectrum of this chromophore lying at the heart of the protein has been measured using the triply resonant DOVESEE-FWM technique. Because of the added electronic enhancement of its DOVE signal, it is possible to home-in on the retinal molecule and pick it out from the surrounding protein.
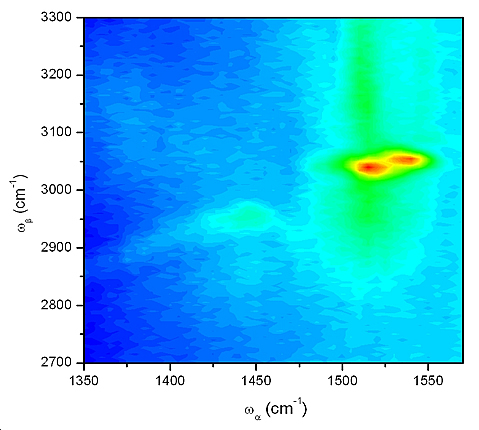
DOVE 2D-IR spectrum of purple membrane; the two peaks represent different retinal isomers.
The above spectrum of bR’s retinal illustrates the ability of the DOVESEE-FWM technique to probe structural changes occurring at the active site of a protein. The two coupled vibrational modes of the retinal molecule that lead to the observed cross peaks have been assigned as the C=C double bond stretch, ν(C=C), and the =C-H single bond stretch, ν(=C-H). Shifts of these peaks are observed for the different retinal isomers that occur throughout the photocycles of bR.

|