|
Exploring the reactivity of tricarbonylchromium complexes of paracyclophanes
|
||
|
Awards and Activities in Chemistry |
||
|
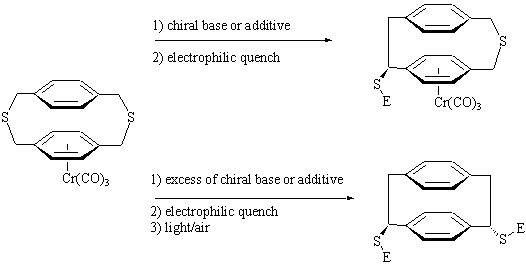
The reactivity of tricarbonylchromium(0) complexes of paracyclophanes has barely been exploited so far, despite its apparent potential in the synthesis of chiral enantioenriched paracyclophanes. From this point of view the following reaction opens a new way to benzylic-substituted [2.2]paracyclophanes via a Wittig-type rearrangement carried out on a (2,11-dithia[3.3]paracyclophane)tricarbonylchromium complex.
Among the potential applications of this reaction are asymmetric deprotonation-Wittig rearrangement sequences employing a non-racemic chiral base or additive. With the right choice of base equivalents, a multiple deprotonation-rearrangement sequence may be envisaged, affording polysubstituted, enantioenriched [2.2]paracyclophanes. These may then be manipulated to generate new ligands for use in asymmetric catalysis.
|