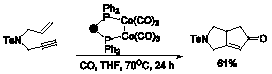|
Pauson Khand Reaction |
||
|
Awards and Activities in Chemistry |
||
|
A significant
amount of research in the group has involved the Pauson-Khand reaction.
Initial efforts in our group in the area of Pauson-Khand catalysis centered on synthesizing and testing the catalytic activity of polymer-supported cobaltcarbonyl Pauson-Khand catalysts.4 Given the well-established use of cobaltcarbonyl derivatives in Pauson-Khand catalysis and the purification and handling advantages of solid-supported materials, we were pleased to find that intramolecular Pauson-Khand substrates could indeed be cyclized.
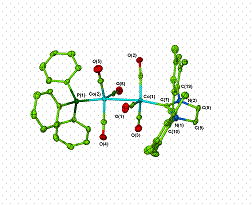
More recent efforts have focused on solution phase (phosphine) carbonyldicobalt(0) complexes. Not only does (PPh3)Co2(CO)7 allow for good yields with both intramolecular and intermolecular Pauson-Khand substrates under mild conditions, but it is also easier to handle and more reliable than the commonly used Co2(CO)8.5 Success with (PPh3)Co2(CO)7 led us to wonder whether N-heterocyclic carbene derivatives of Co2(CO)8 could be synthesized and whether these complexes would promote the Pauson-Khand reaction. Indeed, we did find that these complexes catalyse the cyclocarbonylation of a standard intramolecular Pauson-Khand substrate.6 We were also able to crystallographically characterize two (PPh3)(carbene)Co2(CO)6 complexes, providing the only examples of structurally characterized dicobalthexacarbonyl complexes with two different non-CO ligands.
Asymmetric Pauson-Khand catalysis is also being explored within the group. Good to excellent yields and enantiomeric excesses have been achieved in only 5 h with intramolecular substrates using Co2(CO)8 and axially chiral ligands such as (S)- BINAP.7
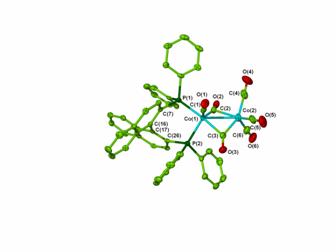
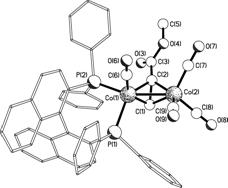
Previous reports8 had suggested that BINAP would bridge the two Co centers but we were able to obtain a crystal structure of (BINAP)Co2(CO)6 showing BINAP chelating a single Co atom. In addition, phosphorus NMR studies have suggested that this complex is an asymmetric Pauson-Khand precatalyst. We are now investigating how the alkyne and alkene portions of Pauson-Khand substrates bind to (BINAP)Co2(CO)6. Preliminary studies have shown that the chelating coordination mode of BINAP does not change after the coordination of an alkyne. Deactivated alkenes will be added to this system to see if the resulting complex can be “frozen” before turnover to the Pauson-Khand product occurs.
We also wish to probe whether Pauson-Khand catalysis can be combined with another catalytic transformation in one pot to carry out tandem transformations. This would allow for the rapid formation of molecules with a significant level of complexity. Finally, intramolecular Pauson-Khand substrates with portions that can be cleaved after catalysis, such as silyl tethers, are being synthesized and evaluated. (1) Khand, I. U.; Knox, G. R.; Pauson, P. L.; Watts, W. E. J. Chem. Soc., Chem. Commun. 1971, 36. (2) Gibson, S. E.; Stevenazzi, A. Angew. Chem. Int. Ed. 2003, 42, 1800. (3) Roberts, S. M.; Santoro, M. G.; Sickle, E. S. J. Chem. Soc., Perkin Trans. 1 2002, 1735. (4) Comely, A. C.; Gibson (née Thomas), S. E.; Hales, N. J. Chem. Commun. 2000, 305. (5) (a) Comely, A. C.; Gibson, S. E.; Stevenazzi, A.; Hales, N. J. Tetrahedron Lett. 2001, 42, 1183. (b) Gibson, S. E.; Johnstone, C.; Stevenazzi, A. Tetrahedron 2002, 58, 4937. (6) Gibson, S. E.; Johnstone, C.; Loch, J. A.; Steed, J. W.; Stevenazzi, A. Organometallics 2003, 22, in press. (7) Gibson, S. E.; Lewis, S. E.; Loch, J. A.; Steed, J. W.; Tozer, M. J. Organometallics 2003, 22, in press. (8) (a) Hiroi, K.; Watanabe, T.; Kawagishi, R.; Abe, I. Tetrahedron Lett. 2000, 41, 891. (b) Hiroi, K.; Watanabe, T.; Kawagishi, R.; Abe, I. Tetrahedron: Asymmetry 2000, 11, 797. (c) Derdau, V.; Laschat, S.; Dix, I.; Jones, P. G. Organometallics 1999, 18, 3859.
|

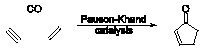
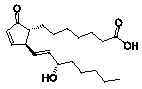 possess
biological activity themselves. Cyclopentenone prostanoids, for example, have
provided promising candidates for anti-inflammatory and antiviral
pharmaceuticals.3
possess
biological activity themselves. Cyclopentenone prostanoids, for example, have
provided promising candidates for anti-inflammatory and antiviral
pharmaceuticals.3