

Medical Research Council Research Fellow, Department of Chemistry, Imperial College London.
Department of Chemistry,
Imperial College,
South Kensington Campus,
Exhibition Road,
London SW7 2AZ
Office : room 835, (020) 7594 5881. Write-up area : room 841, (020) 7594 1169. Laboratory : room 840, (020) 7594 1170. Fax : (020) 7594 5804
Research Interests
MAIN INTEREST: INOSITOL PHOSPHATES, PHOSPHOLIPIDS AND ANALOGUES
The design of such highly charged amphiphilies depends on a deep understanding of polyol protective group chemistry, focussing on the unblocking of a fully protected final product precusor (e.g. 1). During my Ph.D. (with Prof. C.B. Reese, FRS) and post-doctoral research at King's College London (KCL) I developed a general rationale for the synthesis of myo-inositol poly-phosphates and phospholipids based upon protective group strategies originally developed for oligoribonucleotide synthesis. Starting from myo-inositol, the dispositions of permanent acid-labile protective groups (masking the hydroxyls of the target) and base-labile mono- or di-cyanoethylphosphates are carefully controlled. An explicitly two-step total deprotection is then undertaken, using first basic then mildly acidic treatments, to provide poly-phosphoinositides in high yields, with no possibility of phosphoryl migration (Scheme 1).
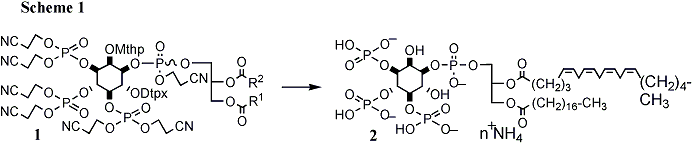
This approach has none of the limitations of earlier hydrogenolytic deprotection strategies, derived from classical carbohydrate chemistry - particularly incompatibility with poly-unsaturated acyl chains (e.g. arachidonoyl, C20:4), phosphorothioates (which are often refractory to phosphatases) or heteroatom groupings (e.g. thiols and azides). Consequently, this research led to the first synthesis of nature-identical mammalian sn-1-stearoyl-2-arachidonoyl phosphatidyl-D-myo-inositol 3,4,5-triphosphate [2, PtdIns(3,4,5)P3] and its diastereomers. These synthetic derivatives provided the first demonstration of the significance of the structure of the fatty acid tails in the phospholipids of the PI-3K growth factor signal transduction pathway (in collaboration with D.Alessi, Dundee University) and were successfully sold commercially to the research community (through Alexis Corp.). Apart form the necessary development of a new general method for the preparation of differentially substituted 1,2- and 2,3-diacyl-sn-glycerols, I also prepared dilinoleoyl (C18:2) phosphatidylinositol 3,4,5-triphosphorothioate and poly-unsaturated phosphatidyl cholines using this approach.
I have continued my interest in inositol chemistry since leaving KCL:-
New head groups: All three naturally occurring isomers of PtdInsP2 are thought to be involved in signalling pathways and are therefore worthy synthetic targets. I have prepared PtdIns(3,5)P2 by modification of my original PtdIns(3,4,5)P3 synthesis. For the other two isomers new partially protected inositol building blocks, compatible with my methodology, were required. In an approach related to that of Reese and Ward [Tetrahedr.Letts., 1987, 28, 2309], a simple high-yielding route to compound 3 has provided the appropriate building block for Ins(1,4,5)P3 and PtdIns(4,5)P2. The preparation of PtdIns(3,4)P2 is more challenging and for this I have been developing the chemistry of xanthen-9,9-yl ketals. A 5,6-O-(2,7-dibromoxanthen-9,9-ylidene)inositol 4 has been carried through to myo-inositol 1,3,4-triphosphate, demonstrating the utility of the new protective groups. Additional regiochemical control on this same building block is expected to enable a synthesis of PtdIns(3,4)P2. We are currently seeking funding (with Prof. R.Templer, Imperial College) to explore the effects of tail unsaturation and/or head-group charge on lipid bilayer curvature, interfacial energetics and the kinetics of inositol phospholipid phosphatases, using these new routes to access the poly-unsaturated phospholipids.
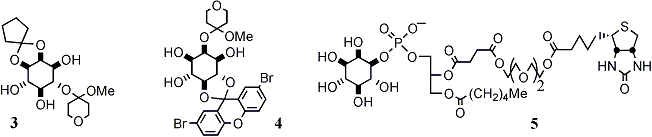
Labelled phosphoinositides: I would like to be able to incorporate fluorescent labels and affinity tags into inositol phospholipid analogues prior to total deprotection. This would ensure quantitive incorporation of the label, with definitive characterisation of the products and wide-ranging flexibility in the choice of linkers/tethers and labels. My synthetic methodology lends itself to this problem since it is compatible with a wide range of functionalities. So far I have tested this proposition with the preparation of biotinylated phosphatidylinositols, replacing the sn-2-fatty acid with a (biotinyl triethylene glycol) adipodiyl ester (5). We are currently investigating other sites of attachment for flourescent labels on the inositol head-group.
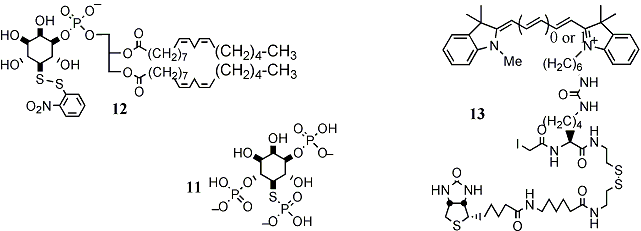
Deoxy hetero-substituted inositides: Despite their being established and effective hydroxide mimics, azido and mercapto inositol phospholipids have not so far been prepared. I have been exploring an approach in which the early stages centre around di-dehydroxylation of differentially tetra-protected inositol trans-diols (6) (Scheme 2). A cis-diol is then re-introduced (8) by catalytic osmylation of the intermediate conduritol (7) and, after selective activation of the axial hydroxyl (9), displacement with either thiolate or azide returns the trans-diequatorial myo-inositol configuration with a heteroatom replacing the natural hydroxyl substituent (10). To date we have prepared myo-inositol 1,4-diphosphate 5-phosphorothiolate (11) and rac-1,2-di-linoleoyl phosphatidylinositol 5-(2-nitrophenyl)disulfide (12). These are being extended towards mercapto- and phosphorothiolo-phospholipids and we will apply a similar strategy to access sulfamido-myo-inositols (Ins-NH-SO3-). I currently hold, in collaboration with R.Woscholski (Imperial College London), a BBSRC grant entitled "Novel Phosphoinositide Reagents for the Study of Phosphoinositide 5-Phosphatases", based on the above research. This interest in modifying features directly attached to the inositol carba-cycle has now led to a new project funded by the MRC entitled "Chemical Genetics with Phosphoinositide Analogues and PH-Domains to Identify Downstream Signalling Elements".
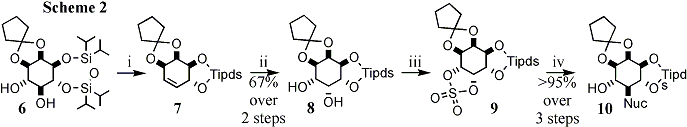
Reagents and conditions : i , Ph3P , I2 , imidazole , toluene , 90 deg.C , 3h ; ii , N-methyl morpholine N-oxide , OsO4 (cat.), acetone - H2O , 2d ; iii , SOCl2 , Et3N , CH2Cl2 , 30min then RuCl3 (cat.), NaIO4 , CH2Cl2 - MeCN - H2O ; iv , NaN3 , DMF , 1h or 2eq. R-SH , 1.2eq. TMG , MeCN , 0 deg.C , 15min.
OTHER PROJECTS
Affinity gel electrophoresis: As part of the LICR’s proteomics effort based on 2D-gel electrophoresis, I have been investigating the concept of a third active dimension, after isoelectric focussing and SDS-poly[acrylamide] gel electrophoresis under non-denaturing conditions. The aim is to electrophorese the protein spots, already spread out on a 2D-gel, perpendicular to the original gel plane up through an increasing gradient of a ligand covalently cross-linked to the poly[acrylamide] matrix. The ligand is chosen to interact with a class of proteins of interest, in this case inositol phospholipid binding proteins, greater retardation indicating the level of affinity. Critical to this approach is efficient co-polymerisation of a ligand-spacer-monomer entity dissolved in the aqueous solution of polymerising acrylamide; the new monomers were proved to be quantitatively retained within poly[acrylamide] gels. I plan to validate this approach to affinity gel electrophoresis (in collaboration with A.Tabor, UCL) using DNA and peptide ligands in 1D-systems prior to applying it to inositol phospholipids.
Fluorescent labels: At KCL I collaborated with Dr. B.V.Smith in studies of chromogenic substrates for pancreatic lipase, prostatic acid phosphatase and N-acetyl glucosaminase. I have recently extended this initial experience with cyanine dyes into the preparation of fluorophores for peptide labelling in proteomics. I have optimised the synthesis of the popular Cy3 and Cy5 dyes, which are a matched pair for 2-dimensional difference gel electrophoresis, and incorporated them into a biotinylated (for affinity purification of the labelled species), reductively-cleavable (for efficient elution from streptavidin beads) iodoacetamide protein label (13). Combining this experience with my knowledge of PEG tethers and peptide chemistry has recently led to a successful proposal to the BBSRC with Drs. A Cass and R. Woscholski (Imperial College London) to develop "lipidomics" tools. This involves the construction of phospholipid binding ligands, labelled with environmentally sensitive fluorophores, assembled in glass supported micro-arrays to rapidly quantify the phospholipid components of complex biological lipid assemblages.
Peptide chemistry: Whilst at UCL, my
group
has been affiliated with that of Dr. A.B.Tabor within UCL Chemistry
Department
which specialises in peptide chemistry. My increasing interest in
heteroatom
chemistry led to participation in a project to synthesise lantibiotics.
The key to preparing this class of cyclic peptides is the synthesis of
a double headed lanthionine monomer, which in our route was derived
from
the nucleophilic displacement of an activated serine beta-O by a
cysteine thiolate anion to give the thioether linkage. Dr. Tabor and I
developed a novel catalyst for Mitsonobu reactions allowing the
displacement
of primary hydroxyls by un-activated simple alkyl thiols. Such
reactions
in the literature only work for electron poor thiols (e.g. thioacetic
acid,
thio glycosides) and this procedure completely avoided epimerisation at
the serine alpha-C.
This experience has since led to a wider
collaboration
including Drs. R. Leatherbarrow and R. Woscholski (IC) in a project
entitled
"Peptide-based Inhibitors for the Phosphoinositide Phosphatases".
OTHER INTERESTS
I am a keen classical pianist (particularly
favouring
Rachmaninoff and Chopin), and also enjoy origami, fell-walking,
gardening
and swimming. I have become a strong hellenophile and speak some modern
Greek poorly. I was president of the University College Chemical
Physical
Society 2001-2.
PUBLICATIONS
1. D.R.Alessi, M.Deak, A.Casamayor, F.B.Caudwell, N.Morrice, D.G.Norman, P.R.J.Gaffney, C.B.Reese, C.N.MacDougall, D.Harbison, A.Ashworth, M.Bownes, "3-Phospho-inositide-dependent Protein Kinase-1 (PDK-1): Structural and Functional Homology with the Drosophila DSTPK61 Kinase", Curr.Biol., 1997, 7, 776-789.
2. D.R.Alessi, S.R.James, C.P.Downes, A.B.Holmes, P.R.J.Gaffney, C.B.Reese, P.Cohen, "Characterisation of a 3-Phos-phoinositide-dependent Protein Kinase which Phosphorylates and Activates Protein Kinase Ba", Curr.Biol., 1997, 7, 261-269.
3. L.R.Stephens, K.Anderson, D.Stokoe, H.Erdjument-Bromage, G.F.Painter, A.B.Holmes, P.R.J.Gaffney, C.B.Reese, F.McCormick, P.Tempst, J.Coadwell, P.T.Hawkins, "Protein Kinase B Kinases that Mediate Phosphatidylinositol 3,4,5-Trisphosphate-dependent Activation of Protein Kinase B", Science, 1998, 279, 710-714.
4. D.Stokoe, L.R.Stephens, T.Copeland, P.R.J.Gaffney, C.B.Reese, G.F.Painter, A.B.Holmes, F.McCormick, P.T.Hawkins, "Dual Role of Phosphatidylinositol-3,4,5-trisphosphate in the Activation of Protein Kinase B", Science, 1997, 277, 567-570.
5. P.R.J.Gaffney, C.B.Reese, "Synthesis of 1-[(1-O-Stearoyl-2-O-arachidonoyl-sn-glycer-3-yl)-phosphoryl]-D-myo-inositol 3,4,5-Trisphosphate [PtdIns(3,4,5)P3] and its Stereoisomers", Bioorg.Med.Chem.Lett., 1997, 7, 3171-3176.
6. P.R.J.Gaffney, C.B.Reese, "Preparation of 2-O-Arachidonoyl-1-O-stearoyl-sn-glycerol and Other Di-O-Acyl Glycerol Derivatives", Tetrahedron Lett., 1997, 38, 2539-2542.
7. P.R.J.Gaffney, L.Changsheng, M.V.Rao, C.B.Reese, J.G.Ward, "Some Substituted 9-Phenylxanthen-9-yl Protecting Groups", J.Chem.Soc., Perkin Trans.1, 1991, 1355-1360.
8. P.Lo Surdo, M.J.Bottomley, A.Arcaro, G.Siegal, G.Panayotou, A.Sankar, P.R.J.Gaffney, A.M.Riley, B.V.L.Potter, M.D.Waterfield, P.C.Driscoll, "Structural and Biochemical Evaluation of the Interaction of the Phosphatidylinositol 3-Kinase p85 Alpha src Homology 2 Domains with Phosphoinositides and Inositol pPolyphosphates", J.Biol.Chem., 1999, 274, 15678-1568.
9. A.Balendran, A.Casamayor, M.Deak, A.Paterson, P.Gaffney, R.Currie, C.P.Downes, D.R.Alessi, "PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2", Curr.Biol., 1999, 9, 393-404.
10. S.Neidle, P.R.J.Gaffney, C.B.Reese, "Myo-inositol 1,3,5-Bicyclic Phosphate", Acta.Cryst.Sect.C, Cryst.Struct.Commun., 1998, 54, 1191-1192.
11. P.R.J.Gaffney, C.B.Reese, "Synthesis of Naturally-occuring Phosphatidylinositol 3,4,5-Trisphosphate [PtdIns(3,4,5)P3] and its Diastereoisomers", J. Chem. Soc., Perkin Trans. 1, 2001, 192-205.
12. C.D.Ellson, S.Gobert-Gosse, K.E.Anderson, K.Davidson, H.Erdjument-Bromage, P.Tempst, J.W.Thuring, M.A.Cooper, Z.-Y. Lim, A.B.Holmes, P.R.J.Gaffney, J.Coadwell, E.R.Chivers, P.T.Hawkins, L.R.Stephens, "PtdIns(3)P Regulates the Neutrophil Oxidase Complex by Binding to the PX Domain of p40phox", Nature Cell Biology, 2001, 3, 679-682.
13. S.Gharbi, P.Gaffney, A.Yang, M.J.Zvelebil, R.Cramer, M.D.Waterfield, J.F.Timms, "Evaluation of Two-dimensional Differential Gel Electrophoresis for Proteomic Expression Analysis of a Model Breast Cancer Cell System", Mol.Cell.Proteomics, 2002, 1.2, 91-98.
14. S. Naaby-Hanse, K.Nagano, P.Gaffney, J.R.W.Masters, R.Cramer, "Proteomics in the Analysis of Prostate Cancer", from methods in Molecular Medicine, 81, Ed.P.J.Russell, P.Jackson, E.A.Kingsley. Humana Press Inc., Totowa, NJ.
15. C.H.Marzabadi, J.E.Anderson, J.Gonzalez-Outeirino, P.R.J.Gaffney , C.G.H.White, D.A.Tocher, L.J.Todaro, "Why are silyl ethers conformationally different from alkyl ethers? Chair-chair conformational equilibria in silyloxycyclohexanes and their dependence on the substituents on silicon. The wider roles of eclipsing, of 1,3-repulsive steric interactions, and of attractive steric interactions", J.Am.Chem.Soc., 2003, 125, 15163-15173.
16. M.F.M.Mustapa, R.Harris, N.Bulic-Subanovic, S.L.Elliott,
S.Bregant,
M.F.A.Groussier, J.Mould, D.Schultz, N.A.L.Chubb, P.R.J.Gaffney,
P.C.Driscoll,
A.B.Tabor,
"Synthesis of orthogonally protected lanthionines", J.Org.Chem.,
2003, 68, 8185-8192.