 VRML Molecules
VRML Molecules VRML Movie
VRML Movie Reaction Pathway Animations
Reaction Pathway Animations
In recent years, Computational Quantum Chemistry has become a powerful tool for the study of many chemical reactions. Through the combined use of increasingly faster computers and sophisticated programs, chemists are now able to study various chemical properties, such as molecular structure and chemical reactivity.
The corresponding emergence of visualization programs is allowing chemists to better treat the quantum results, and consequently leads to a better understanding of the molecular properties. Moreover, visualization allows chemists to present their results in a highly comprehensible manner.
Some difficulties can arise, however, since all the theoretical calculations imply the use of the "supermolecule approach" technique, that is, the treatment of the two (or n) chemical moieties as a supermolecule by the program. For instance, the calculation of the Diels-Alder reaction path requires that the the diene and the dienophile be included in the same input file. In other words, all the calculated molecular properties belong to the "supermolecule" and not to the individual moieties.
This perturbation theory approach - the effect of the interaction of the MO of the two components as a perturbation on each other - has been applied to a wide range of well known Diels-Alder reactions [2] (with C, Z and X-side groups [3]) in order to better study the regioselectivity of such reactions.
VRML is basically a 3D metaphore of HTML text. A VRML viewer such as SGI WebSpace acts as a helper application for World Wide Web (WWW) browsers. EyeChem itself was extended into a VRML viewer[4].
The complete EyeChem process of splitting each MERP into two moieties and preparing all the visualizations for this paper can be summarized as follows:
1) The HOMO/LUMO interactions of the diene and the dieneophile in their transition state (TS) were visualized and saved as both gif images and VRML files.
2) The reaction coordinate output was read in and, for only one reaction coordinate, the moiety to be replaced by dummy atoms was selected.
3) New MOPAC-93 input files for each reaction coordinate were automatically generated with one of the moities in each input file containing the dummy atoms.
4) A single-point MOPAC-93 calculation on each input file generated the graph files.
5) Steps 2-4 were repeated for the other moiety.
One example QuickTime movie of a reaction pathway was prepared. A QuickTime movie, although better than a static image, conveys only one particular and unalterable viewpoint. However movies are often created and viewed in EyeChem as a sequence of 3D geometry files. Such 3D movies can be viewed from any perspective and are thus preferable to the conventional formats. One example 3D VRML movie was created for this paper. It is a sequence of VRML files viewable in WebSpace
 VRML Molecules
VRML Molecules VRML Movie
VRML Movie Reaction Pathway Animations
Reaction Pathway Animations
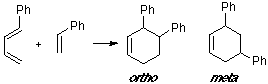
Example of a 1-C-substituted diene with a C-substituted dienophile, having an observed ortho/meta adduct ratio of 8:1. In this case the MO overlapping in the ortho TS is slightly better than in the meta TS.
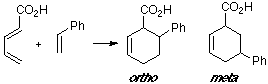
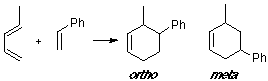
Example of a 1-Z-substituted diene with a C-substituted dienophile, having an observed ortho/meta adduct ratio of 6:1. No great differences are shown from the visualization of the MO's.
Example of a 1-X-substituted diene with a C-substituted dienophile. Here the observed ratio is slightly favorable to the ortho adduct.
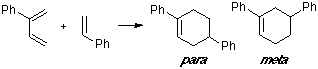
Example of a 2-C-substituted diene with a C-substituted dienophile, having an observed para/meta adduct ratio of 20:1. It can be seen that the overlap contribution of the phenyl group is greater for the para TS than for the meta TS.
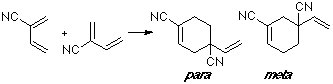
Example of a 2-Z-substituted diene with a C-substituted dienophile. In this case we are considering the dimerization of 2-cyano-butadiene, with the double bond (C1-C2) of one moiety as a dienophile. The observed product is the para adduct, that shows the better overlap.
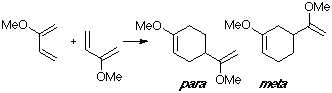
Example of a 2-X-substituted diene with a C-substituted dienophile. As in the previous reaction, we consider the dimerization of 2-methoxy-butadiene, with the para adduct being the observed one. The para TS clearly shows the interaction of the 2-methoxy group in the overlap.
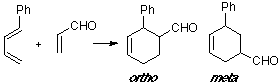
Example of a 1-C-substituted diene with a Z-substituted dienophile, where the observed product is an ortho adduct. The ortho TS shows the only suitable overlap.
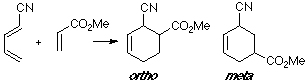
Example of a 1-Z-substituted diene with a Z-substituted dienophile. In this case, where two Z groups exist, both overlappings are very close.
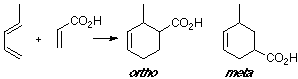
Example of a 1-X-substituted diene with a Z-substituted dienophile. Same case in our earlier example (see Abstract), where the most suitable overlap is related to the ortho adduct.
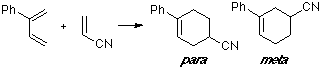
Example of a 2-C-substituted diene with a Z-substituted dienophile, with an observed para/meta ratio of 4:1. No substantial differences can be seen between the MO of both TS.
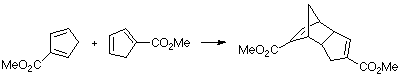
Example of a 2-Z-substituted diene with a Z-substituted dienophile. This case is especially remarkable due to the very rapid 1,5-sigmatropic hydrogen shifts of the cyclopentadiene. This leads to the presence of all three isomers in the reaction and could give a wide range of products. We only present the MO of the TS leading to the observed product.
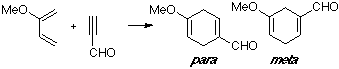
Example of a 2-X-substituted diene with a Z-substituted dienophile, in this case a substituted acetylene. Although the observed product is the para adduct, only small differences can be seen between the MO of both TS structures.
In studying Perturbation Theory, chemists can obtain a much better understanding of chemical reactions by visualizing the MO of each reactant along the MERP. We are aware that key factors, in addition MO overlapping, must be taken into account when trying to explain regioselectivity. The importance of 3D visualization, however, is clearly evident. Moreover, new sophisticated VRML browsers as WWW helper applications provide a novel chemical tool useful for research and educational purposes.
1.. MOPAC-93-93: J. J. P. Stewart, Fujitsu Limited, Tokyo, Japan, 1993. Available from Quantum Chemistry Program Exchange, University of Indiana, Bloomington, IN.
2.. All examples are taken from Fleming, Ian. "Frontier Orbitals and Organic Chemical Reactions" John Wiley & Sons.
3.. Terminology is:
4.. O. Casher and H. S. Rzepa, "Chemical Collaboratories using World-Wide Web Servers and EyeChem Based Viewers", J. Mol. Graphics, to be published.